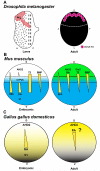
Figure 1. Retinas are patterned in stochastic/regionalized, regionalized, and ordered mosaics
A1) Schematic of the Drosophila melanogaster (fruit fly) PR mosaic (not to scale). D: Dorsal, V: Ventral, A: Anterior, P: Posterior. A2) Schematic of a Drosophila ommatidium. A3) Schematic of a pale ommatidium. A4) Schematic of a yellow ommatidium. A5) Schematic of a dorsal third yellow ommatidium. A6) Schematic of a dorsal rim ommatidium. A7) Whole-mount immunostain of a Drosophila retina showing the stochastic distribution of ommatidial subtypes. A8) Immunostain showing Rhodopsin 1 (Rh1) expression in the outer PRs. A9) Immunostain showing the stochastic patterning of Rh3 and Rh4 in a section of the Drosophila retina. A10) Immunostain showing the stochastic patterning of Rh5 and Rh6 in a section of the Drosophila retina. A11) Immunostain of the dorsal third of the Drosophila retina, showing coexpression of Rh3 and Rh4 in dorsal third yR7s. A12) Immunostain of the dorsal rim of the Drosophila retina, showing expression of Rh3 in R7s and R8s. B1) Schematic of the Danio rerio (zebrafish) PR mosaic (not to scale). D: Dorsal, V: Ventral, A: Anterior, P: Posterior. B2) Schematic side view of a single unit of the zebrafish retinal pattern. B3) Schematic showing the overlapping, regionalized expression patterns of zebrafish LWS and RH2 opsin subtypes (not to scale). 1: Inner central/dorsal area, 2: Outer central/dorsal area, 3: Inner periphery/ventral area, 4: Outer periphery/ventral area. B4) Immunostain of a section of the zebrafish cone mosaic. Reprinted from Progress in Retinal and Eye Research, Volume 42, M. Hoon, H. Okawa, L. Della Santina, R.O. Wong, Functional architecture of the retina: Development and disease, Pages 44-84, Copyright (2014), with permission from Elsevier. B5) Immunostain of a section of the zebrafish rod mosaic. Reprinted from Developmental Biology, Volume 258, J.M. Fadool, Development of a rod photoreceptor mosaic revealed in transgenic zebrafish, Pages 277-290, Copyright (2003), with permission from Elsevier. C1) Schematic of the Gallus gallus domesticus (chicken) PR mosaic (not to scale). 1: area centralis, 2: dorsal rod free zone, 3: dorsal rod zone, 4: central meridian, 5: ventral rod rich zone. C2) The chicken has five different types of cone cells: red, green, blue, violet, and double cones. Type A double cones contain an auxiliary cone lacking an oil droplet. Type B double cones both have oil droplets. Images adapted from Wai et al., 2006 and Santiago Ramon y Cajal, 2000(46, 254). C3) Light microscope image of oil droplets in the chicken retina. Adapted from Figure 1b from Kram et al., 2010(43). D1) Schematic of the Mus musculus (mouse) PR mosaic (not to scale). D2-D5) Labeled depiction and immunostaining of mouse PRs. Rods shown in yellow (D2), S-cones in blue (D3), M-cones in green (D4), and S/M-cones in blue/green (D5). D6) Immunostain of a whole-mount mouse retina. Green: M-opsin. Blue: S-opsin. D7) Pseudocolored DIC section of whole-mount mouse retina, showing cone and rod distribution. Rods shown in yellow. Blue and green are arbitrarily chosen to represent S- and M-cones, respectively, but each cell could express S-opsin only, M-opsin only, or both S- and M-opsins. Adapted from Jeon et al. 1998(58). Copyright 1998, http://www.jneurosci.org/content/18/21/8936.long, under Creative Commons Attribution 4.0 International Public License and Disclaimer of Warranties (http://creativecommons.org/licenses/by/4.0/legalcode). E1) Schematic of the Homo sapiens (human) PR mosaic (not to scale). 1: foveola, 2: fovea, 3: macula, 4: posterior pole, 5: peripheral rim. E2-E5) Labeled depiction of human PRs. E2: rod, E3: S-cone, E4: L-cone, E5: M-cone. E6) Pseudocolored adaptive optics image of the human fovea. Blue: S-cones, Red: L-cones, Green: M-cones. Adapted from Figure 8B of Williams et al., 2011(77). Copyright 2011, http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3189497/, DOI 10.1016/j.visres.2011.05.002, under Creative Commons Attribution 4.0 International Public License and Disclaimer of Warranties (http://creativecommons.org/licenses/by/4.0/legalcode). E7) Pseudocolored image of human cones in the posterior pole. Yellow: rods, Blue: S-cones. Red and green are arbitrarily chosen to represent L- and M-cones, respectively, but each cell could be either red or green. Adapted from Curcio et al., 1991(67). Copyright 1991, http://onlinelibrary.wiley.com/doi/10.1002/cne.903120411/abstract, DOI 10.1002/cne.903120411, under Creative Commons Attribution 4.0 International Public License and Disclaimer of Warranties (http://creativecommons.org/licenses/by/4.0/legalcode). Note: In Danio rerio and Mus musculus, the optic disc is located temporal to the central retina, and in Gallus gallus domesticus and Homo sapiens retinas, it is located temporal to the foveal center. This area is devoid of photoreceptors and is not represented in the included mosaics.