Stress-induced adaptive islet cell identity changes
- PMID: 27615136
- PMCID: PMC5021189
- DOI: 10.1111/dom.12726
Stress-induced adaptive islet cell identity changes
Abstract
The different forms of diabetes mellitus differ in their pathogenesis but, ultimately, they are all characterized by progressive islet β-cell loss. Restoring the β-cell mass is therefore a major goal for future therapeutic approaches. The number of β-cells found at birth is determined by proliferation and differentiation of pancreatic progenitor cells, and it has been considered to remain mostly unchanged throughout adult life. Recent studies in mice have revealed an unexpected plasticity in islet endocrine cells in response to stress; under certain conditions, islet non-β-cells have the potential to reprogram into insulin producers, thus contributing to restore the β-cell mass. Here, we discuss the latest findings on pancreas and islet cell plasticity upon physiological, pathological and experimental conditions of stress. Understanding the mechanisms involved in cell reprogramming in these models will allow the development of new strategies for the treatment of diabetes, by exploiting the intrinsic regeneration capacity of the pancreas.
Keywords: adaptive cell plasticity; cell conversion; cell dedifferentiation; cell fate change; cell identity; cell reprogramming; cell transdifferentiation; diabetes; diabetes treatment; islet; pancreas; transgenic.
© 2016 John Wiley & Sons Ltd.
Figures
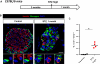


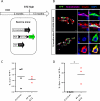
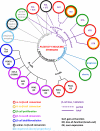
References
-
- Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. - PubMed
-
- Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–2567. - PubMed
-
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

