Mutational signatures of ionizing radiation in second malignancies
- PMID: 27615322
- PMCID: PMC5027243
- DOI: 10.1038/ncomms12605
Mutational signatures of ionizing radiation in second malignancies
Abstract
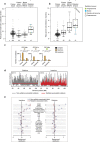
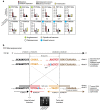
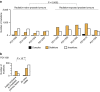
Ionizing radiation is a potent carcinogen, inducing cancer through DNA damage. The signatures of mutations arising in human tissues following in vivo exposure to ionizing radiation have not been documented. Here, we searched for signatures of ionizing radiation in 12 radiation-associated second malignancies of different tumour types. Two signatures of somatic mutation characterize ionizing radiation exposure irrespective of tumour type. Compared with 319 radiation-naive tumours, radiation-associated tumours carry a median extra 201 deletions genome-wide, sized 1-100 base pairs often with microhomology at the junction. Unlike deletions of radiation-naive tumours, these show no variation in density across the genome or correlation with sequence context, replication timing or chromatin structure. Furthermore, we observe a significant increase in balanced inversions in radiation-associated tumours. Both small deletions and inversions generate driver mutations. Thus, ionizing radiation generates distinctive mutational signatures that explain its carcinogenic potential.
Figures
References
-
- March H. C. Leukemia in radiologists. Radiology 43, 275–278 (1944).
-
- Preston D. L. et al.. Radiation effects on breast cancer risk: a pooled analysis of eight cohorts. Radiat. Res. 158, 220–235 (2002). - PubMed
-
- Ronckers C. M., Doody M. M., Lonstein J. E., Stovall M. & Land C. E. Multiple diagnostic X-rays for spine deformities and risk of breast cancer. Cancer Epidemiol. Biomarkers Prev. 17, 605–613 (2008). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

