Decreased PD-1 Expression on CD8 Lymphocyte Subsets and Increase in CD8 Tscm Cells in Children with HIV Receiving Raltegravir
- PMID: 27615375
- PMCID: PMC5312622
- DOI: 10.1089/AID.2016.0108
Decreased PD-1 Expression on CD8 Lymphocyte Subsets and Increase in CD8 Tscm Cells in Children with HIV Receiving Raltegravir
Abstract
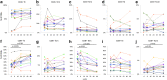
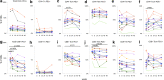
We investigated the effect of combination antiretroviral therapy (cART) on immune recovery, particularly on the percentages of PD-1-positive cells within the major leukocyte subsets. Cryopreserved peripheral blood mononuclear cells and plasma samples collected longitudinally from a subset of 13 children and adolescents (between 9.7 and 18.2 years old) who were enrolled in the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1066 were used for this study. Immunophenotyping by flow cytometry was performed to determine the effect of raltegravir-containing cART regimen on the distribution of leukocyte populations, on the expression of PD-1 on T cell subpopulations, and on the expression of well-established markers of T cell activation (CD38 and HLA-DR) on CD8 T cells. C reactive protein (CRP), lipopolysaccharide (LPS), IL-6, and soluble CD163 were assayed in plasma samples by an enzyme-linked immunosorbent assay. Plasma viral loads were decreased in all subjects (by an average of 2.9 log units). The cART regimen, including raltegravir, induced changes in CD8 T cell subsets, consistent with an effective antiretroviral outcome and improved immunologic status, including increased percentages of CD8 stem cell memory T cells (Tscm). The percentages of CD8 PD-1-positive cells decreased significantly as compared with baseline levels. Among the proinflammatory markers measured in plasma, sCD163 showed a decline that was associated with cART. cART therapy, including raltegravir, over 48 weeks in children is associated with immune restoration, consistent with effective antiretroviral therapy, namely decreased percentages of PD-1+ CD8+ T cells, an increase in CD8 Tscm cells, and decreased levels of sCD163.
Keywords: HIV; T cell immunity; antiretroviral therapy; clinical trials for antivirals; integrase inhibitors/drug discovery.
Conflict of interest statement
No competing financial interests exist.
Figures



References
-
- Lennox JL, DeJesus E, Lazzarin A, et al. : Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: A multicentre, double-blind randomised controlled trial. Lancet (London, England) 2009;374:796–806 - PubMed
-
- Rockstroh JK, DeJesus E, Lennox JL, et al. : Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: Final 5-year results from STARTMRK. J Acquir Immune Defic Syndr 2013;63:77–85 - PubMed
-
- De Castro N, Braun J, Charreau I, et al. : Switch from enfuvirtide to raltegravir in virologically suppressed multidrug-resistant HIV-1-infected patients: A randomized open-label trial. Clin Infect Dis 2009;49:1259–1267 - PubMed
-
- Steigbigel RT, Cooper DA, Kumar PN, et al. : Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med 2008;359:339–354 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

