Efficacy and safety of ibrutinib in Japanese patients with relapsed or refractory mantle cell lymphoma
- PMID: 27616553
- PMCID: PMC5198949
- DOI: 10.1111/cas.13076
Efficacy and safety of ibrutinib in Japanese patients with relapsed or refractory mantle cell lymphoma
Abstract
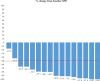
In this multicenter, single-arm, phase II study, the efficacy and safety of ibrutinib were examined in Japanese patients with relapsed or refractory mantle cell lymphoma (MCL). Patients (age ≥20 years) with relapsed or refractory MCL who had progressed after receiving at least one prior treatment regimen, were enrolled. Patients were treated with oral ibrutinib (560 mg once daily; 28-day cycle) until disease progression (or relapse), unacceptable toxicity, or study end. The primary end-point was overall response rate. Secondary end-points included duration of response (DOR), time to response, progression-free survival (PFS), overall survival, and safety. Of the 16 patients who received treatment, 5 patients discontinued the study (progressive disease, 4; sepsis, 1). Median duration of ibrutinib exposure was 6.5 months (range, 2.8-8.3 months). The overall response rate was 87.5% (90% confidence interval, 65.6-97.7; complete response = 2 [12.5%]; partial response = 12 [75.0%]). Median time to response for all responders (n = 14) was 1.8 months (range, 0.7-5.3 months). The median DOR and PFS were not estimable due to censoring (range: DOR, 1.1-6.4+ months; PFS, 2.8-8.0+ months). Overall survival data were immature due to the limited observation period. A total of 8/16 patients (50%) had at least one grade 3 adverse event (AE), and 5 (31.3%) patients reported serious AEs. The most commonly reported AEs were diarrhea and stomatitis (37.5% each), platelet count decrease (31.3%), and anemia (25%). Overall, orally administered single agent ibrutinib was efficacious with an acceptable safety profile in Japanese patients with relapsed or refractory MCL. Clinical trial registration NCT02169180 (ClinicalTrials.gov).
Keywords: Efficacy; ibrutinib; mantle cell lymphoma; overall response rate; safety.
© 2016 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
References
-
- Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood 2009; 114: 1469–76. - PubMed
-
- Lymphoma Study Group of Japanese . The world health organization classification of malignant lymphomas in japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int 2000; 50: 696–702. - PubMed
-
- Dreyling M, Geisler C, Hermine O et al Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol Off J Eur Soc Med Oncol ESMO 2014; 25(Suppl. 3): iii83–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

