PI3Kδ and primary immunodeficiencies
- PMID: 27616589
- PMCID: PMC5291318
- DOI: 10.1038/nri.2016.93
PI3Kδ and primary immunodeficiencies
Abstract
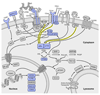
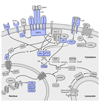
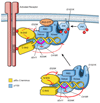
Primary immunodeficiencies are inherited disorders of the immune system, often caused by the mutation of genes required for lymphocyte development and activation. Recently, several studies have identified gain-of-function mutations in the phosphoinositide 3-kinase (PI3K) genes PIK3CD (which encodes p110δ) and PIK3R1 (which encodes p85α) that cause a combined immunodeficiency syndrome, referred to as activated PI3Kδ syndrome (APDS; also known as p110δ-activating mutation causing senescent T cells, lymphadenopathy and immunodeficiency (PASLI)). Paradoxically, both loss-of-function and gain-of-function mutations that affect these genes lead to immunosuppression, albeit via different mechanisms. Here, we review the roles of PI3Kδ in adaptive immunity, describe the clinical manifestations and mechanisms of disease in APDS and highlight new insights into PI3Kδ gleaned from these patients, as well as implications of these findings for clinical therapy.
Conflict of interest statement
C.L.L. collaborates with Novartis. A.C., S.N., A.M.C. and K.O. collaborate with and receive research funding from GSK. K.O. has received consultancy or speaker fees from Karus Pharmaceutical, Merck, Gilead and Incyte.
Figures

References
Highlighted references
-
-
Angulo, I. et al. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science 342, 866-71 (2013).
- [ Together with reference 4, the first papers showing that activated mutations in PIK3CD cause primary immunodeficency (APDS/PASLI). ]
-
-
-
Deau, M.C. et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest 124, 3923-8 (2014).
- [ Together with reference 5, the first papers showing that activating mutations in PIK3R1 cause a primary immunodeficiency (APDS-2/PASLI-R) ]
-
-
-
Lucas, C.L. et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol 15, 88-97 (2014).
-
-
-
Lucas, C.L. et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med 211, 2537-47 (2014).
-
-
-
Elkaim, E. et al. Clinical and immunological phenotype associated with activated PI3-kinase delta syndrome 2 (APDS2 / PASLI-R1) - A cohort study. Journal of Allergy and Clinical Immunology (2016).
- [ Clinical and immunological features associated with activating PIK3R1 mutations: a survey of 36 patients. ]
-
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/B/000C0409/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- UL1 TR001863/TR/NCATS NIH HHS/United States
- MR/M012328/1/MRC_/Medical Research Council/United Kingdom
- BBS/E/B/000C0407/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 095691/Z/11/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

