Involvement of mitochondrial permeability transition pore (mPTP) in cardiac arrhythmias: Evidence from cyclophilin D knockout mice
- PMID: 27616659
- PMCID: PMC5127715
- DOI: 10.1016/j.ceca.2016.09.001
Involvement of mitochondrial permeability transition pore (mPTP) in cardiac arrhythmias: Evidence from cyclophilin D knockout mice
Abstract
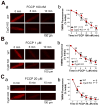
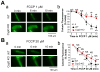
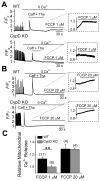
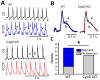
In the present study, we have used a genetic mouse model that lacks cyclophilin D (CypD KO) to assess the cardioprotective effect of mitochondrial permeability transition pore (mPTP) inhibition on Ca2+ waves and Ca2+ alternans at the single cell level, and cardiac arrhythmias in whole-heart preparations. The protonophore carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) caused mitochondrial membrane potential depolarization to the same extent in cardiomyocytes from both WT and CypD KO mice, however, cardiomyocytes from CypD KO mice exhibited significantly less mPTP opening than cardiomyocytes from WT mice (p<0.05). Consistent with these results, FCCP caused significant increases in CaW rate in WT cardiomyocytes (p<0.05) but not in CypD KO cardiomyocytes. Furthermore, the incidence of Ca2+ alternans after treatment with FCCP and programmed stimulation was significantly higher in WT cardiomyocytes (11 of 13), than in WT cardiomyocytes treated with CsA (2 of 8; p<0.05) or CypD KO cardiomyocytes (2 of 10; p<0.01). (Pseudo-)Lead II ECGs were recorded from ex vivo hearts. We observed ST-T-wave alternans (a precursor of lethal arrhythmias) in 5 of 7 WT hearts. ST-T-wave alternans was not seen in CypD KO hearts (n=5) and in only 1 of 6 WT hearts treated with CsA. Consistent with these results, WT hearts exhibited a significantly higher average arrhythmia score than CypD KO (p<0.01) hearts subjected to FCCP treatment or chemical ischemia-reperfusion (p<0.01). In conclusion, CypD deficiency- induced mPTP inhibition attenuates CaWs and Ca2+ alternans during mitochondrial depolarization, and thereby protects against arrhythmogenesis in the heart.
Keywords: Arrhythmias; Calcium; Cyclophilin D; Heart; Mitochondria; mPTP.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


References
-
- Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem. 1992;267:8834–8839. - PubMed
-
- Dedkova EN, Blatter LA. Mitochondrial Ca2+ and the heart. Cell Calcium. 2008;44:77–91. - PubMed
-
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

