Glycocalyx and its involvement in clinical pathophysiologies
- PMID: 27617097
- PMCID: PMC5017018
- DOI: 10.1186/s40560-016-0182-z
Glycocalyx and its involvement in clinical pathophysiologies
Abstract
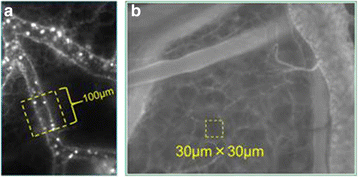
Vascular hyperpermeability is a frequent intractable feature involved in a wide range of diseases in the intensive care unit. The glycocalyx (GCX) seemingly plays a key role to control vascular permeability. The GCX has attracted the attention of clinicians working on vascular permeability involving angiopathies, and several clinical approaches to examine the involvement of the GCX have been attempted. The GCX is a major constituent of the endothelial surface layer (ESL), which covers most of the surface of the endothelial cells and reduces the access of cellular and macromolecular components of the blood to the surface of the endothelium. It has become evident that this structure is not just a barrier for vascular permeability but contributes to various functions including signal sensing and transmission to the endothelium. Because GCX is a highly fragile and unstable layer, the image had been only obtained by conventional transmission electron microscopy. Recently, advanced microscopy techniques have enabled direct visualization of the GCX in vivo, most of which use fluorescent-labeled lectins that bind to specific disaccharide moieties of glycosaminoglycan (GAG) chains. Fluorescent-labeled solutes also enabled to demonstrate vascular leakage under the in vivo microscope. Thus, functional analysis of GCX is advancing. A biomarker of GCX degradation has been clinically applied as a marker of vascular damage caused by surgery. Fragments of the GCX, such as syndecan-1 and/or hyaluronan (HA), have been examined, and their validity is now being examined. It is expected that GCX fragments can be a reliable diagnostic or prognostic indicator in various pathological conditions. Since GCX degradation is strongly correlated with disease progression, pharmacological intervention to prevent GCX degradation has been widely considered. HA and other GAGs are candidates to repair GCX; further studies are needed to establish pharmacological intervention. Recent advancement of GCX research has demonstrated that vascular permeability is not regulated by simple Starling's law. Biological regulation of vascular permeability by GCX opens the way to develop medical intervention to control vascular permeability in critical care patients.
Keywords: Endothelial surface layer; Glycocalyx; Heparan sulfate; Hyaluronan; Lectin; Leukocyte; Sepsis; Starling’s law; Syndecan-1; Vascular permeability.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

