Inside out: Bone marrow adipose tissue as a source of circulating adiponectin
- PMID: 27617171
- PMCID: PMC5014002
- DOI: 10.1080/21623945.2016.1149269
Inside out: Bone marrow adipose tissue as a source of circulating adiponectin
Abstract
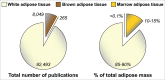
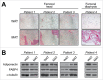
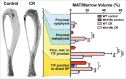
The adipocyte-derived hormone adiponectin mediates beneficial cardiometabolic effects, and hypoadiponectinemia is a biomarker for increased metabolic and cardiovascular risk. Indeed, circulating adiponectin decreases in obesity and insulin-resistance, likely because of impaired production from white adipose tissue (WAT). Conversely, lean states such as caloric restriction (CR) are characterized by hyperadiponectinemia, even without increased adiponectin production from WAT. The reasons underlying this paradox have remained elusive, but our recent research suggests that CR-associated hyperadiponectinemia derives from an unexpected source: bone marrow adipose tissue (MAT). Herein, we elaborate on this surprising discovery, including further discussion of potential mechanisms influencing adiponectin production from MAT; additional evidence both for and against our conclusions; and observations suggesting that the relationship between MAT and adiponectin might extend beyond CR. While many questions remain, the burgeoning study of MAT promises to reveal further key insights into MAT biology, both as a source of adiponectin and beyond.
Keywords: adiponectin; anorexia nervosa; bone marrow adipose tissue; caloric restriction; lipodystrophy; obesity; white adipose tissue.
Figures


References
-
- Romacho T, Elsen M, Rohrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta physiologica 2014; 210:733-53; PMID:24495317; http://dx.doi.org/10.1111/apha.12246 - DOI - PubMed
-
- Scherer PEE. Adiponectin: basic and clinical aspects [Special Issue]. Best Pract Res Clin Endocrinol Metab 2014; 28:1-130; PMID:24417940; http://dx.doi.org/10.1016/j.beem.2013.11.004 - DOI - PubMed
-
- Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab 2013; 2:133-41; PMID:24049728; http://dx.doi.org/10.1016/j.molmet.2013.04.001 - DOI - PMC - PubMed
-
- Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, et al.. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab 2014; 20:368-75; PMID:24998914; http://dx.doi.org/10.1016/j.cmet.2014.06.003 - DOI - PMC - PubMed
-
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al.. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999; 257:79-83; PMID:10092513; http://dx.doi.org/10.1006/bbrc.1999.0255 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
