Atomic-scale compositional mapping reveals Mg-rich amorphous calcium phosphate in human dental enamel
- PMID: 27617291
- PMCID: PMC5014466
- DOI: 10.1126/sciadv.1601145
Atomic-scale compositional mapping reveals Mg-rich amorphous calcium phosphate in human dental enamel
Abstract
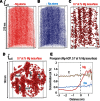
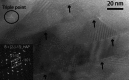
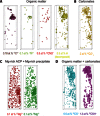
Human dental enamel, the hardest tissue in the body, plays a vital role in protecting teeth from wear as a result of daily grinding and chewing as well as from chemical attack. It is well established that the mechanical strength and fatigue resistance of dental enamel are derived from its hierarchical structure, which consists of periodically arranged bundles of hydroxyapatite (HAP) nanowires. However, we do not yet have a full understanding of the in vivo HAP crystallization process that leads to this structure. Mg(2+) ions, which are present in many biological systems, regulate HAP crystallization by stabilizing its precursor, amorphous calcium phosphate (ACP), but their atomic-scale distribution within HAP is unknown. We use atom probe tomography to provide the first direct observations of an intergranular Mg-rich ACP phase between the HAP nanowires in mature human dental enamel. We also observe Mg-rich elongated precipitates and pockets of organic material among the HAP nanowires. These observations support the postclassical theory of amelogenesis (that is, enamel formation) and suggest that decay occurs via dissolution of the intergranular phase. This information is also useful for the development of more accurate models to describe the mechanical behavior of teeth.
Keywords: Human dental enamel; Mg-rich amorphous phase; atom probe tomography; tooth decay.
Figures


References
-
- World Health Organization (WHO), “Oral health” (Fact sheet no. 318, WHO, Geneva, 2012); www.who.int/mediacentre/factsheets/fs318/en/.
-
- Meckel A. H., Griebstein W. J., Neal R. J., Structure of mature human dental enamel as observed by electron microscopy. Arch. Oral Biol. 10, 775–782 (1965). - PubMed
-
- Eastoe J. E., Organic matrix of tooth enamel. Nature 187, 411–412 (1960). - PubMed
-
- Kerebel B., Daculsi G., Kerebel L. M., Ultrastructural studies of enamel crystallites. J. Dent. Res. 58, 844–851 (1979). - PubMed
-
- Daculsi G., Menanteau J., Kerebel L. M., Mitre D., Length and shape of enamel crystals. Calcif. Tissue Int. 36, 550–555 (1984). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

