Self-repair promotes microtubule rescue
- PMID: 27617929
- PMCID: PMC5045721
- DOI: 10.1038/ncb3406
Self-repair promotes microtubule rescue
Abstract
The dynamic instability of microtubules is characterized by slow growth phases stochastically interrupted by rapid depolymerizations called catastrophes. Rescue events can arrest the depolymerization and restore microtubule elongation. However, the origin of these rescue events remains unexplained. Here we show that microtubule lattice self-repair, in structurally damaged sites, is responsible for the rescue of microtubule growth. Tubulin photo-conversion in cells revealed that free tubulin dimers can incorporate along the shafts of microtubules, especially in regions where microtubules cross each other, form bundles or become bent due to mechanical constraints. These incorporation sites appeared to act as effective rescue sites ensuring microtubule rejuvenation. By securing damaged microtubule growth, the self-repair process supports a mechanosensitive growth by specifically promoting microtubule assembly in regions where they are subjected to physical constraints.
Conflict of interest statement
Competing financial interests. The authors have no competing financial interests.
Figures
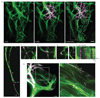
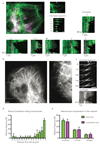
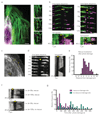
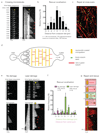


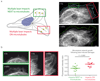
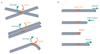
Comment in
-
Cytoskeleton: Patching up microtubule growth.Nat Rev Mol Cell Biol. 2016 Nov;17(11):677. doi: 10.1038/nrm.2016.131. Epub 2016 Sep 21. Nat Rev Mol Cell Biol. 2016. PMID: 27649881 No abstract available.
-
Cell Biology: Microtubule Collisions to the Rescue.Curr Biol. 2016 Dec 19;26(24):R1287-R1289. doi: 10.1016/j.cub.2016.11.010. Curr Biol. 2016. PMID: 27997842
References
-
- Mimori-Kiyosue Y. Shaping microtubules into diverse patterns: Molecular connections for setting up both ends. Cytoskeleton. 2011;68:603–618. - PubMed
-
- Stiess M, et al. Axon extension occurs independently of centrosomal microtubule nucleation. Science. 2010;327:704–7. - PubMed
-
- Wadsworth P. Regional regulation of microtubule dynamics in polarized, motile cells. Cell Motil Cytoskeleton. 1999;42:48–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

