Anti-Ephrin Type-B Receptor 2 (EphB2) and Anti-Three Prime Histone mRNA EXonuclease 1 (THEX1) Autoantibodies in Scleroderma and Lupus
- PMID: 27617966
- PMCID: PMC5019431
- DOI: 10.1371/journal.pone.0160283
Anti-Ephrin Type-B Receptor 2 (EphB2) and Anti-Three Prime Histone mRNA EXonuclease 1 (THEX1) Autoantibodies in Scleroderma and Lupus
Abstract
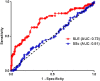
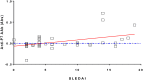
In a pilot ProtoArray analysis, we identified 6 proteins out of 9483 recognized by autoantibodies (AAb) from patients with systemic sclerosis (SSc). We further investigated the 6 candidates by ELISA on hundreds of controls and patients, including patients with Systemic Lupus Erythematosus (SLE), known for high sera reactivity and overlapping AAb with SSc. Only 2 of the 6 candidates, Ephrin type-B receptor 2 (EphB2) and Three prime Histone mRNA EXonuclease 1 (THEX1), remained significantly recognized by sera samples from SSc compared to controls (healthy or with rheumatic diseases) with, respectively, 34% versus 14% (P = 2.10-4) and 60% versus 28% (P = 3.10-8). Above all, EphB2 and THEX1 revealed to be mainly recognized by SLE sera samples with respectively 56%, (P = 2.10-10) and 82% (P = 5.10-13). As anti-EphB2 and anti-THEX1 AAb were found in both diseases, an epitope mapping was realized on each protein to refine SSc and SLE diagnosis. A 15-mer peptide from EphB2 allowed to identify 35% of SLE sera samples (N = 48) versus only 5% of any other sera samples (N = 157), including SSc sera samples. AAb titers were significantly higher in SLE sera (P<0.0001) and correlated with disease activity (p<0.02). We could not find an epitope on EphB2 protein for SSc neither on THEX1 for SSc or SLE. We showed that patients with SSc or SLE have AAb against EphB2, a protein involved in angiogenesis, and THEX1, a 3'-5' exoribonuclease involved in histone mRNA degradation. We have further identified a peptide from EphB2 as a specific and sensitive tool for SLE diagnosis.
Conflict of interest statement
There are two patents to declare: EP14305364.3 Diagnosis method for Lupus; EP15306481.1 EPHB2 Polypeptides and uses thereof for the diagnosis and treatment of Lupus. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures






Similar articles
-
Circulating anticentromere CENP-A and CENP-B antibodies in patients with diffuse and limited systemic sclerosis, systemic lupus erythematosus, and rheumatoid arthritis.J Rheumatol. 2000 Jan;27(1):142-8. J Rheumatol. 2000. PMID: 10648030
-
Naturally occurring and disease-associated auto-antibodies against topoisomerase I: a fine epitope mapping study in systemic sclerosis and systemic lupus erythematosus.Int Immunol. 2009 Apr;21(4):415-22. doi: 10.1093/intimm/dxp008. Epub 2009 Feb 11. Int Immunol. 2009. PMID: 19211583
-
Autoantibodies in Serum of Systemic Scleroderma Patients: Peptide-Based Epitope Mapping Indicates Increased Binding to Cytoplasmic Domains of CXCR3.Front Immunol. 2018 Mar 22;9:428. doi: 10.3389/fimmu.2018.00428. eCollection 2018. Front Immunol. 2018. PMID: 29623076 Free PMC article.
-
Olfactory function in systemic lupus erythematosus and systemic sclerosis. A longitudinal study and review of the literature.Autoimmun Rev. 2018 Apr;17(4):405-412. doi: 10.1016/j.autrev.2018.02.002. Epub 2018 Feb 11. Autoimmun Rev. 2018. PMID: 29444467 Review.
-
Bicaudal D2 is a novel autoantibody target in systemic sclerosis that shares a key epitope with CENP-A but has a distinct clinical phenotype.Autoimmun Rev. 2018 Mar;17(3):267-275. doi: 10.1016/j.autrev.2018.01.006. Epub 2018 Jan 31. Autoimmun Rev. 2018. PMID: 29369808 Review.
Cited by
-
EphB2 Receptor Promotes Dermal Fibrosis in Systemic Sclerosis.Arthritis Rheumatol. 2024 Aug;76(8):1303-1316. doi: 10.1002/art.42858. Epub 2024 May 15. Arthritis Rheumatol. 2024. PMID: 38589317 Free PMC article.
-
Association of autoantibodies with the IFN signature and NETosis in patients with systemic lupus erythematosus.J Transl Autoimmun. 2024 Jun 20;9:100246. doi: 10.1016/j.jtauto.2024.100246. eCollection 2024 Dec. J Transl Autoimmun. 2024. PMID: 39027720 Free PMC article.
-
Amino acid sequence homology between thyroid autoantigens and central nervous system proteins: Implications for the steroid-responsive encephalopathy associated with autoimmune thyroiditis.J Clin Transl Endocrinol. 2021 Nov 6;26:100274. doi: 10.1016/j.jcte.2021.100274. eCollection 2021 Dec. J Clin Transl Endocrinol. 2021. PMID: 34849350 Free PMC article.
References
-
- Allanore Y, Cabane J, Mouthon L. Sclérodermies In: Med-line, editor. Classification et évaluation des sclérodermies. Paris: 2007.
-
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis and rheumatism. 2013;65(11):2737–47. 10.1002/art.38098 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

