Investigation into Deep Brain Stimulation Lead Designs: A Patient-Specific Simulation Study
- PMID: 27618109
- PMCID: PMC5039468
- DOI: 10.3390/brainsci6030039
Investigation into Deep Brain Stimulation Lead Designs: A Patient-Specific Simulation Study
Abstract
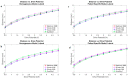
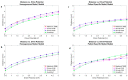
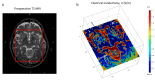
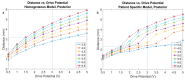
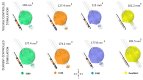
New deep brain stimulation (DBS) electrode designs offer operation in voltage and current mode and capability to steer the electric field (EF). The aim of the study was to compare the EF distributions of four DBS leads at equivalent amplitudes (3 V and 3.4 mA). Finite element method (FEM) simulations (n = 38) around cylindrical contacts (leads 3389, 6148) or equivalent contact configurations (leads 6180, SureStim1) were performed using homogeneous and patient-specific (heterogeneous) brain tissue models. Steering effects of 6180 and SureStim1 were compared with symmetric stimulation fields. To make relative comparisons between simulations, an EF isolevel of 0.2 V/mm was chosen based on neuron model simulations (n = 832) applied before EF visualization and comparisons. The simulations show that the EF distribution is largely influenced by the heterogeneity of the tissue, and the operating mode. Equivalent contact configurations result in similar EF distributions. In steering configurations, larger EF volumes were achieved in current mode using equivalent amplitudes. The methodology was demonstrated in a patient-specific simulation around the zona incerta and a "virtual" ventral intermediate nucleus target. In conclusion, lead design differences are enhanced when using patient-specific tissue models and current stimulation mode.
Keywords: brain model; deep brain stimulation (DBS); electric field; electrode design; finite element method; neuron model; patient-specific; steering; zona incerta (ZI).
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Hemm S., Mennessier G., Vayssiere N., Cif L., El Fertit H., Coubes P. Deep brain stimulation in movement disorders: Stereotactic coregistration of two-dimensional electrical field modeling and magnetic resonance imaging. J. Neurosurg. 2005;103:949–955. doi: 10.3171/jns.2005.103.6.0949. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

