Structural Basis for Negative Allosteric Modulation of GluN2A-Containing NMDA Receptors
- PMID: 27618671
- PMCID: PMC5033714
- DOI: 10.1016/j.neuron.2016.08.014
Structural Basis for Negative Allosteric Modulation of GluN2A-Containing NMDA Receptors
Abstract
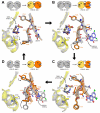
NMDA receptors mediate excitatory synaptic transmission and regulate synaptic plasticity in the central nervous system, but their dysregulation is also implicated in numerous brain disorders. Here, we describe GluN2A-selective negative allosteric modulators (NAMs) that inhibit NMDA receptors by stabilizing the apo state of the GluN1 ligand-binding domain (LBD), which is incapable of triggering channel gating. We describe structural determinants of NAM binding in crystal structures of the GluN1/2A LBD heterodimer, and analyses of NAM-bound LBD structures corresponding to active and inhibited receptor states reveal a molecular switch in the modulatory binding site that mediate the allosteric inhibition. NAM binding causes displacement of a valine in GluN2A and the resulting steric effects can be mitigated by the transition from glycine bound to apo state of the GluN1 LBD. This work provides mechanistic insight to allosteric NMDA receptor inhibition, thereby facilitating the development of novel classes NMDA receptor modulators as therapeutic agents.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





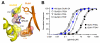

References
-
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J. Comp. Neurol. 1994;347:150–160. - PubMed
-
- Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, Negri M, Andreetta F, Senar-Sancho SA, Guiral L, et al. Identification and characterization of novel NMDA receptor antagonists selective for NR2A-over NR2B-containing receptors. J. Pharmacol. Exp. Ther. 2010;335:636–644. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

