Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release
- PMID: 27618677
- PMCID: PMC5033724
- DOI: 10.1016/j.neuron.2016.08.017
Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release
Abstract
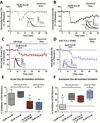
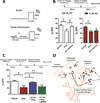
Muscarinic receptors represent a promising therapeutic target for schizophrenia, but the mechanisms underlying the antipsychotic efficacy of muscarinic modulators are not well understood. Here, we report that activation of M4 receptors on striatal spiny projection neurons results in a novel form of dopaminergic regulation resulting in a sustained depression of striatal dopamine release that is observed more than 30 min after removal of the muscarinic receptor agonist. Furthermore, both the M4-mediated sustained inhibition of dopamine release and the antipsychotic-like efficacy of M4 activators were found to require intact signaling through CB2 cannabinoid receptors. These findings highlight a novel mechanism by which striatal cholinergic and cannabinoid signaling leads to sustained reductions in dopaminergic transmission and concurrent behavioral effects predictive of antipsychotic efficacy.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. - PubMed
-
- Bubser M, Bridges TM, Dencker D, Gould RW, Grannan M, Noetzel MJ, Lamsal A, Niswender CM, Daniels JS, Poslusney MS, et al. Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem Neurosci. 2014;5:920–942. - PMC - PubMed
-
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 2003;17:1403–1410. - PubMed
-
- Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, Gowrishankar R, Kelm ND, Damon S, Bridges TM, et al. Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology. 2014;39:1578–1593. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

