Efficacy and Safety of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia and LDL-C of 160 mg/dl or Higher
- PMID: 27618825
- PMCID: PMC5055560
- DOI: 10.1007/s10557-016-6685-y
Efficacy and Safety of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia and LDL-C of 160 mg/dl or Higher
Abstract
Purpose: Even with statins and other lipid-lowering therapy (LLT), many patients with heterozygous familial hypercholesterolemia (heFH) continue to have elevated low-density lipoprotein cholesterol (LDL-C) levels. ODYSSEY HIGH FH (NCT01617655) assessed the efficacy and safety of alirocumab, a proprotein convertase subtilisin/kexin type 9 monoclonal antibody, versus placebo in patients with heFH and LDL-C ≥ 160 mg/dl despite maximally tolerated statin ± other LLT.
Methods: Patients were randomized to subcutaneous alirocumab 150 mg or placebo every 2 weeks (Q2W) for 78 weeks. The primary endpoint was percent change in LDL-C from baseline to week 24.
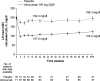
Results: Mean baseline LDL-C levels were 196.3 mg/dl in the alirocumab (n = 71) and 201.0 mg/dl in the placebo groups (n = 35). Significant mean (standard error [SE]) reductions in LDL-C from baseline to week 24 were observed with alirocumab (-45.7 [3.5] %) versus placebo (-6.6 [4.9] %), a difference of -39.1 (6.0) % (P < 0.0001). Absolute mean (SE) LDL-C levels were reduced from baseline by 90.8 (6.7) mg/dl with alirocumab at week 24, with reductions maintained to week 78. Treatment-emergent adverse events were generally comparable between groups. Injection-site reactions were more frequent in the alirocumab group (8.3 %) versus placebo (5.7 %); most were mild in severity and did not result in study medication discontinuation.
Conclusions: In patients with heFH and very high LDL-C baseline levels despite maximally tolerated statin ± other LLT, alirocumab 150 mg Q2W demonstrated significant reductions in LDL-C levels with 41 % of patients achieving predefined LDL-C goals. Alirocumab was generally well tolerated.
Keywords: Alirocumab; Cardiovascular disease prevention; Cholesterol-lowering drugs; Familial hypercholesterolemia; LDL-C; PCSK9.
Conflict of interest statement
Compliance with Ethical Standards Conflict of Interests Henry N. Ginsberg has received research support from Genzyme (Sanofi), Sanofi and Regeneron Pharmaceuticals, Inc., has served as a consultant on advisory boards for Sanofi and Regeneron Pharmaceuticals, Inc., and has served as a consultant for Amarin, Amgen, AstraZeneca, Bristol - Myers Squibb, GlaxoSmithKline, ISIS, Kowa, Merck, Novartis, and Pfizer. Daniel J. Rader has served as a consultant for Alnylam, Pfizer, Novartis and Sanofi. Frederick J. Raal has received research grants from Amgen, Sanofi and Regeneron Pharmaceuticals, Inc., has served on and received honoraria for a Speakers Bureau and consultant/advisory boards for Amgen, Sanofi, Regeneron Pharmaceuticals, Inc., Pfizer, and AstraZeneca. John R. Guyton has received research support from Sanofi, Regeneron Pharmaceuticals, Inc., Abbott, Genzyme/Sanofi, GlaxoSmithKline, Amarin Pharma, and Amgen. He has served as a consultant for Sanofi, Regeneron Pharmaceuticals, Inc. and Novella and has received speaker fees from Merck. Christelle Lorenzato and Marie T. Baccara-Dinet are employees of Sanofi. Robert Pordy is an employee of Regeneron Pharmaceuticals, Inc. Erik Stroes has served as a consultant/advisory board member for MSD, Amgen, Sanofi, Regeneron Pharmaceuticals, Inc., ISIS pharmaceuticals and Torrent. Research Involving Human Participants The study was performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki and all applicable amendments laid down by the World Medical Assemblies and the International Conference Harmonization guidelines for Good Clinical Practice. The protocol was approved by the local institutional review board and independent ethics committee at each site. Informed Consent All patients provided written informed consent prior to participation.
Figures

Comment in
-
PCSK9: the Critical Role of Familial Hypercholesterolemia from Discovery to Benefit for all : Editorial to: "Efficacy and Safety of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia and LDL-C of 160 mg/Dl or Higher" by Henry N. Ginsberg et Al.Cardiovasc Drugs Ther. 2016 Oct;30(5):427-431. doi: 10.1007/s10557-016-6690-1. Cardiovasc Drugs Ther. 2016. PMID: 27669716 No abstract available.
References
-
- Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European atherosclerosis society. Eur Heart J. 2013;34:3478–3390a. doi: 10.1093/eurheartj/eht273. - DOI - PMC - PubMed
-
- Reiner Z, Catapano AL, De BG, et al. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European atherosclerosis society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

