The analysis of heterotaxy patients reveals new loss-of-function variants of GRK5
- PMID: 27618959
- PMCID: PMC5020398
- DOI: 10.1038/srep33231
The analysis of heterotaxy patients reveals new loss-of-function variants of GRK5
Abstract
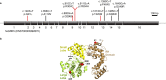
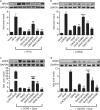
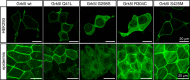
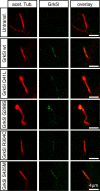
G protein-coupled receptor kinase 5 (GRK5) is a regulator of cardiac performance and a potential therapeutic target in heart failure in the adult. Additionally, we have previously classified GRK5 as a determinant of left-right asymmetry and proper heart development using zebrafish. We thus aimed to identify GRK5 variants of functional significance by analysing 187 individuals with laterality defects (heterotaxy) that were associated with a congenital heart defect (CHD). Using Sanger sequencing we identified two moderately frequent variants in GRK5 with minor allele frequencies <10%, and seven very rare polymorphisms with minor allele frequencies <1%, two of which are novel variants. Given their evolutionarily conserved position in zebrafish, in-depth functional characterisation of four variants (p.Q41L, p.G298S, p.R304C and p.T425M) was performed. We tested the effects of these variants on normal subcellular localisation and the ability to desensitise receptor signalling as well as their ability to correct the left-right asymmetry defect upon Grk5l knockdown in zebrafish. While p.Q41L, p.R304C and p.T425M responded normally in the first two aspects, neither p.Q41L nor p.R304C were capable of rescuing the lateralisation phenotype. The fourth variant, p.G298S was identified as a complete loss-of-function variant in all assays and provides insight into the functions of GRK5.
Figures

References
-
- Lin A. E., Ticho B. S., Houde K., Westgate M. N. & Holmes L. B. Heterotaxy: associated conditions and hospital-based prevalence in newborns. Genet Med 2, 157–172, doi: 10.109700125817-200005000-00002 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

