Development and evaluation of real-time loop-mediated isothermal amplification assay for rapid detection of cystic echinococcosis
- PMID: 27619674
- PMCID: PMC5020552
- DOI: 10.1186/s12917-016-0809-2
Development and evaluation of real-time loop-mediated isothermal amplification assay for rapid detection of cystic echinococcosis
Abstract
Background: Cystic echinococcosis (CE) or hydatidosis, caused by the larval stage of Echinococcus granulosus (EG)-complex, is a neglected parasitic disease of public health importance. The disease is endemic in many African and Mediterranean countries including the Sudan. The objective of the present study was to develop and evaluate a real-time loop-mediated isothermal amplification (LAMP) assay for simple and rapid detection of CE in humans and domestic live stock in Sudan.
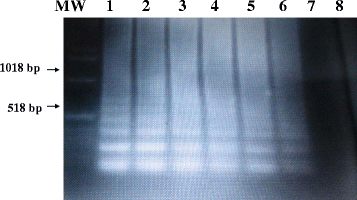
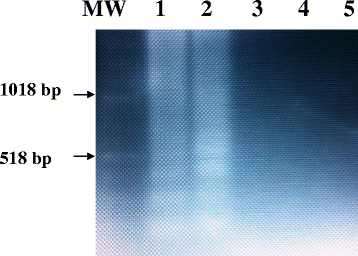
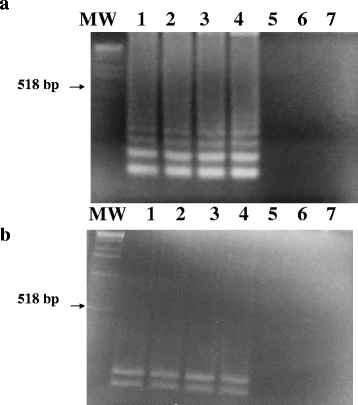
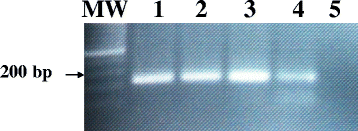
Methods: A set of six LAMP primers, designed from the mitochondrial NADH-1 gene of EG cattle strain of genotype 5 (G5), was used as a target for LAMP assay. The assay was performed at a constant temperature (63 °C), with a real-time follow-up using a LightCycler and fluorochrome dye. Following amplification cycles in a simple water bath, LAMP products were observed for color change by naked eye and were visualized under UV light source using agarose gel electrophoresis.
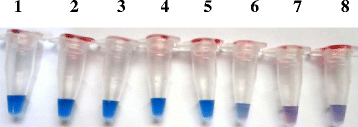
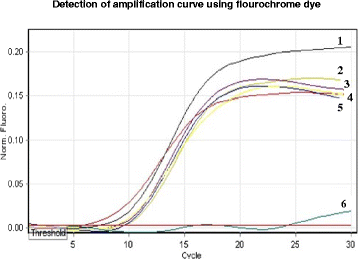
Results: The real-time LAMP assay identified a variety of hydatid cysts strains recovered in the Sudan, including Echinococcus canadenses (G6) and Echinococcus ortleppi (G5). Real-time LAMP positive results were detected by the presence of an amplification curve, whereas negative results were indicated by absence of fluorescence detection. Positive LAMP results appeared as a bluish-colored reaction as observed by naked eye, whereas negative LAMP results were observed as purple-colored reaction. The sensitivity studies indicated that the LAMP assay detected as little as a 10 fg of parasite DNA. There was 100 % agreement between results of the LAMP assay and our previously described nested PCR when testing 10-fold serial dilution of DNA extracted from EG-complex hydatid cyst. However, there was no cross-reactivity with other parasites including cysticercus bovis, Fasciola gigantica, and Schistosoma bovis and nucleic acid free samples.
Conclusion: The developed LAMP assay would be expected to prove highly significant in epidemiological surveys of CE in developing countries or areas of resource-poor settings for both ease of use and cost.
Keywords: Cystic echinococcosis; Echinococcus granulosus-complex; Hydatid cysts; LAMP; Sudan.
Figures

References
-
- Abdel Malek E. Check-list of helminth parasites of domestic animals in the Sudan. Indian Vet J. 1959;36:28–286.
-
- Eisa AM, Mustfa AA, Soliman KN. Preliminary report on cysticercosis and hydatidosis in the Southern Sudan. Sud J Vet Sc Anim Husb. 1962;3:97–102.
-
- Eisa AM, El Khawat SE, Slepnev NK. Proceedings of the 8th Veterinary Conference. Khartoum: Livestock and animal production in the Sudan; 1977. A survey of parasites of dogs in Khartoum province.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

