Toll-Like Receptor 2 Recognizes Orientia tsutsugamushi and Increases Susceptibility to Murine Experimental Scrub Typhus
- PMID: 27620720
- PMCID: PMC5116716
- DOI: 10.1128/IAI.00185-16
Toll-Like Receptor 2 Recognizes Orientia tsutsugamushi and Increases Susceptibility to Murine Experimental Scrub Typhus
Abstract
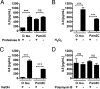
Scrub typhus is a potentially lethal infection that is caused by the obligate intracellular bacterium Orientia tsutsugamushi The roles of Toll-like receptor 2 (TLR2) and TLR4 in innate recognition of O. tsutsugamushi have not been elucidated. By overexpression of TLR2 or TLR4 in HEK293 cells, we demonstrated that TLR2, but not TLR4, recognizes heat-stable compounds of O. tsutsugamushi that were sensitive to treatment with sodium hydroxide, hydrogen peroxide, and proteinase K. TLR2 was required for the secretion of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) by dendritic cells. In an intradermal mouse infection model, TLR2-deficient mice did not show impaired control of bacterial growth or reduced survival. Moreover, after intraperitoneal infection, TLR2-deficient mice were even more resistant to lethal infection than C57BL/6 wild-type mice, which showed stronger symptoms and lower survival rates during the convalescent phase. Compared to the time of reduction of bacterial loads in TLR2-deficient mice, the reduction of bacterial loads in infected organs was accelerated in wild-type mice. The higher mortality of wild-type mice was associated with increased concentrations of serum alkaline phosphatase but not aspartate aminotransferase. The transcription of mRNA for TNF-α and IL-6 decreased more rapidly in peritoneum samples from wild-type mice than in those from TLR2-deficient mice and was therefore not a correlate of increased susceptibility. Thus, although TLR2 is an important mediator of the early inflammatory response, it is dispensable for protective immunity against O. tsutsugamushi Increased susceptibility to O. tsutsugamushi infection in TLR2-competent mice rather suggests a TLR2-related immunopathologic effect.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
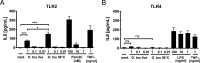
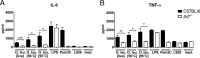
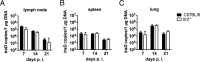

Comment in
-
Suspected Mycoplasma Contamination in the Study "Toll-Like Receptor 2 Recognizes Orientia tsutsugamushi and Increases Susceptibility to Murine Experimental Scrub Typhus".Infect Immun. 2017 Aug 18;85(9):e00269-17. doi: 10.1128/IAI.00269-17. Print 2017 Sep. Infect Immun. 2017. PMID: 28821640 Free PMC article. No abstract available.
-
Reply to Tantibhedhyangkul et al., 'Suspected Mycoplasma Contamination in the Study "Toll-Like Receptor 2 Recognizes Orientia tsutsugamushi and Increases Susceptibility to Murine Experimental Scrub Typhus"'.Infect Immun. 2017 Aug 18;85(9):e00326-17. doi: 10.1128/IAI.00326-17. Print 2017 Sep. Infect Immun. 2017. PMID: 28821641 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

