Vegfc acts through ERK to induce sprouting and differentiation of trunk lymphatic progenitors
- PMID: 27621059
- PMCID: PMC5087638
- DOI: 10.1242/dev.137901
Vegfc acts through ERK to induce sprouting and differentiation of trunk lymphatic progenitors
Erratum in
-
Correction: Vegfc acts through ERK to induce sprouting and differentiation of trunk lymphatic progenitors.Development. 2017 Feb 1;144(3):531. doi: 10.1242/dev.148569. Development. 2017. PMID: 28143848 Free PMC article. No abstract available.
Abstract
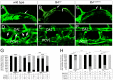
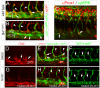
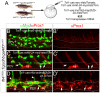
Vascular endothelial growth factor C (Vegfc) activates its receptor, Flt4, to induce lymphatic development. However, the signals that act downstream of Flt4 in this context in vivo remain unclear. To understand Flt4 signaling better, we generated zebrafish bearing a deletion in the Flt4 cytoplasmic domain that eliminates tyrosines Y1226 and 1227. Embryos bearing this deletion failed to initiate sprouting or differentiation of trunk lymphatic vessels and did not form a thoracic duct. Deletion of Y1226/7 prevented ERK phosphorylation in lymphatic progenitors, and ERK inhibition blocked trunk lymphatic sprouting and differentiation. Conversely, endothelial autonomous ERK activation rescued lymphatic sprouting and differentiation in flt4 mutants. Interestingly, embryos bearing the Y1226/7 deletion formed a functional facial lymphatic network enabling them to develop normally to adulthood. By contrast, flt4 null larvae displayed hypoplastic facial lymphatics and severe lymphedema. Thus, facial lymphatic vessels appear to be the first functional lymphatic network in the zebrafish, whereas the thoracic duct is initially dispensable for lymphatic function. Moreover, distinct signaling pathways downstream of Flt4 govern lymphatic morphogenesis and differentiation in different anatomical locations.
Keywords: Flt4; Lymphatic; TALEN; Vegfc; Zebrafish.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures




References
-
- Boscolo E., Coma S., Luks V. L., Greene A. K., Klagsbrun M., Warman M. L. and Bischoff J. (2015). AKT hyper-phosphorylation associated with PI3K mutations in lymphatic endothelial cells from a patient with lymphatic malformation. Angiogenesis 18, 151-162. 10.1007/s10456-014-9453-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

