Molecular Architecture of Contactin-associated Protein-like 2 (CNTNAP2) and Its Interaction with Contactin 2 (CNTN2)
- PMID: 27621318
- PMCID: PMC5104938
- DOI: 10.1074/jbc.M116.748236
Molecular Architecture of Contactin-associated Protein-like 2 (CNTNAP2) and Its Interaction with Contactin 2 (CNTN2)
Abstract
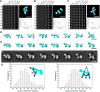
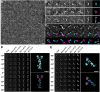
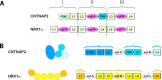
Contactin-associated protein-like 2 (CNTNAP2) is a large multidomain neuronal adhesion molecule implicated in a number of neurological disorders, including epilepsy, schizophrenia, autism spectrum disorder, intellectual disability, and language delay. We reveal here by electron microscopy that the architecture of CNTNAP2 is composed of a large, medium, and small lobe that flex with respect to each other. Using epitope labeling and fragments, we assign the F58C, L1, and L2 domains to the large lobe, the FBG and L3 domains to the middle lobe, and the L4 domain to the small lobe of the CNTNAP2 molecular envelope. Our data reveal that CNTNAP2 has a very different architecture compared with neurexin 1α, a fellow member of the neurexin superfamily and a prototype, suggesting that CNTNAP2 uses a different strategy to integrate into the synaptic protein network. We show that the ectodomains of CNTNAP2 and contactin 2 (CNTN2) bind directly and specifically, with low nanomolar affinity. We show further that mutations in CNTNAP2 implicated in autism spectrum disorder are not segregated but are distributed over the whole ectodomain. The molecular shape and dimensions of CNTNAP2 place constraints on how CNTNAP2 integrates in the cleft of axo-glial and neuronal contact sites and how it functions as an organizing and adhesive molecule.
Keywords: cell adhesion; cell surface receptor; contactin; contactin-associated protein like; neuropsychiatric disorders; protein-protein interaction; single particle analysis; structural biology; synapse; synaptic organizer.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Scott-Van Zeeland A. A., Abrahams B. S., Alvarez-Retuerto A. I., Sonnenblick L. I., Rudie J. D., Ghahremani D., Mumford J. A., Poldrack R. A., Dapretto M., Geschwind D. H., and Bookheimer S. Y. (2010) Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Sci. Transl. Med. 2, 56ra80 - PMC - PubMed
-
- Buchner D. A., Geisinger J. M., Glazebrook P. A., Morgan M. G., Spiezio S. H., Kaiyala K. J., Schwartz M. W., Sakurai T., Furley A. J., Kunze D. L., Croniger C. M., and Nadeau J. H. (2012) The juxtaparanodal proteins CNTNAP2 and TAG1 regulate diet-induced obesity. Mamm. Genome 23, 431–442 - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

