CD8 T cell-mediated killing of orexinergic neurons induces a narcolepsy-like phenotype in mice
- PMID: 27621438
- PMCID: PMC5047186
- DOI: 10.1073/pnas.1603325113
CD8 T cell-mediated killing of orexinergic neurons induces a narcolepsy-like phenotype in mice
Abstract
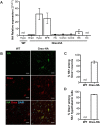
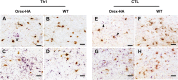
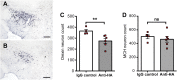
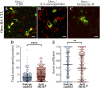
Narcolepsy with cataplexy is a rare and severe sleep disorder caused by the destruction of orexinergic neurons in the lateral hypothalamus. The genetic and environmental factors associated with narcolepsy, together with serologic data, collectively point to an autoimmune origin. The current animal models of narcolepsy, based on either disruption of the orexinergic neurotransmission or neurons, do not allow study of the potential autoimmune etiology. Here, we sought to generate a mouse model that allows deciphering of the immune mechanisms leading to orexin(+) neuron loss and narcolepsy development. We generated mice expressing the hemagglutinin (HA) as a "neo-self-antigen" specifically in hypothalamic orexin(+) neurons (called Orex-HA), which were transferred with effector neo-self-antigen-specific T cells to assess whether an autoimmune process could be at play in narcolepsy. Given the tight association of narcolepsy with the human leukocyte antigen (HLA) HLA-DQB1*06:02 allele, we first tested the pathogenic contribution of CD4 Th1 cells. Although these T cells readily infiltrated the hypothalamus and triggered local inflammation, they did not elicit the loss of orexin(+) neurons or clinical manifestations of narcolepsy. In contrast, the transfer of cytotoxic CD8 T cells (CTLs) led to both T-cell infiltration and specific destruction of orexin(+) neurons. This phenotype was further aggravated upon repeated injections of CTLs. In situ, CTLs interacted directly with MHC class I-expressing orexin(+) neurons, resulting in cytolytic granule polarization toward neurons. Finally, drastic neuronal loss caused manifestations mimicking human narcolepsy, such as cataplexy and sleep attacks. This work demonstrates the potential role of CTLs as final effectors of the immunopathological process in narcolepsy.
Keywords: CD8 T cells; autoimmunity; narcolepsy; orexin; sleep disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

