Stac3 has a direct role in skeletal muscle-type excitation-contraction coupling that is disrupted by a myopathy-causing mutation
- PMID: 27621462
- PMCID: PMC5047181
- DOI: 10.1073/pnas.1612441113
Stac3 has a direct role in skeletal muscle-type excitation-contraction coupling that is disrupted by a myopathy-causing mutation
Abstract
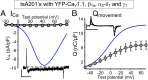
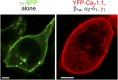
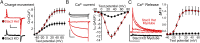
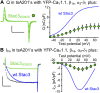
In skeletal muscle, conformational coupling between CaV1.1 in the plasma membrane and type 1 ryanodine receptor (RyR1) in the sarcoplasmic reticulum (SR) is thought to underlie both excitation-contraction (EC) coupling Ca(2+) release from the SR and retrograde coupling by which RyR1 increases the magnitude of the Ca(2+) current via CaV1.1. Recent work has shown that EC coupling fails in muscle from mice and fish null for the protein Stac3 (SH3 and cysteine-rich domain 3) but did not establish the functional role of Stac3 in the CaV1.1-RyR1 interaction. We investigated this using both tsA201 cells and Stac3 KO myotubes. While confirming in tsA201 cells that Stac3 could support surface expression of CaV1.1 (coexpressed with its auxiliary β1a and α2-δ1 subunits) and the generation of large Ca(2+) currents, we found that without Stac3 the auxiliary γ1 subunit also supported membrane expression of CaV1.1/β1a/α2-δ1, but that this combination generated only tiny Ca(2+) currents. In Stac3 KO myotubes, there was reduced, but still substantial CaV1.1 in the plasma membrane. However, the CaV1.1 remaining in Stac3 KO myotubes did not generate appreciable Ca(2+) currents or EC coupling Ca(2+) release. Expression of WT Stac3 in Stac3 KO myotubes fully restored Ca(2+) currents and EC coupling Ca(2+) release, whereas expression of Stac3W280S (containing the Native American myopathy mutation) partially restored Ca(2+) currents but only marginally restored EC coupling. We conclude that membrane trafficking of CaV1.1 is facilitated by, but does not require, Stac3, and that Stac3 is directly involved in conformational coupling between CaV1.1 and RyR1.
Keywords: L-type Ca2+ channels; Stac3 protein; excitation–contraction coupling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Tanabe T, Beam KG, Powell JA, Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336(6195):134–139. - PubMed
-
- Takeshima H, et al. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994;369(6481):556–559. - PubMed
-
- Arikkath J, Campbell KP. Auxiliary subunits: Essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13(3):298–307. - PubMed
-
- Freise D, et al. Absence of the γ subunit of the skeletal muscle dihydropyridine receptor increases L-type Ca2+ currents and alters channel inactivation properties. J Biol Chem. 2000;275(19):14476–14481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

