Nonproliferative and Proliferative Lesions of the Rat and Mouse Skeletal Tissues (Bones, Joints, and Teeth)
- PMID: 27621538
- PMCID: PMC5013709
- DOI: 10.1293/tox.29.3S-2
Nonproliferative and Proliferative Lesions of the Rat and Mouse Skeletal Tissues (Bones, Joints, and Teeth)
Abstract
The INHAND (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) Project (www.toxpath.org/inhand.asp) is an initiative of the Societies of Toxicological Pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP) and North America (STP) to develop an internationally accepted nomenclature for proliferative and nonproliferative lesions in laboratory animals. The purpose of this publication is to provide a standardized nomenclature for classifying microscopic lesions observed in the skeletal tissues and teeth of laboratory rats and mice, with color photomicrographs illustrating examples of many common lesions. The standardized nomenclature presented in this document is also available on the internet (http://www.goreni.org/). Sources of material were databases from government, academic and industrial laboratories throughout the world.
Keywords: bone; diagnostic criteria; diagnostic pathology; joint; nomenclature; skeletal system; tooth.
Figures
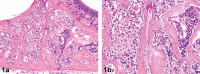

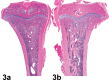

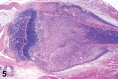



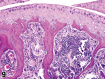


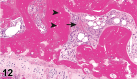
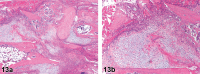







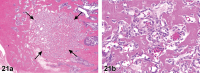


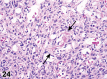
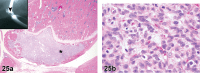

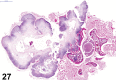


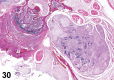
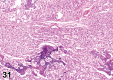

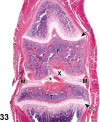



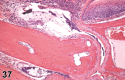
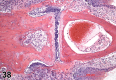

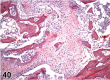
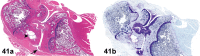



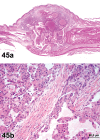
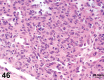
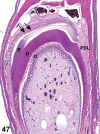







References
-
- Ahmad N, and Ruch JV. Comparison of growth and cell proliferation kinetics during mouse molar odontogenesis in vivo and in vitro. Cell Tissue Kinet. 20: 319–329. 1987. - PubMed
-
- Aigner T, Cook JL, Gerwin N, Glasson SS, Laverty S, Little CB, McIlwraith W, and Kraus VB. Histopathology atlas of animal model systems - overview of guiding principles. Osteoarthritis Cartilage. 18(Suppl 3): S2–S6. 2010. - PubMed
-
- Albassam MA, Wojcinski ZW, Barsoum NJ, and Smith GS. Spontaneous fibro-osseous proliferative lesions in the sternums and femurs of B6C3F1 mice. Vet Pathol. 28: 381–388. 1991. - PubMed
-
- Alvarez J, Horton J, Sohn P, and Serra R. The perichondrium plays an important role in mediating the effects of TGF-beta1 on endochondral bone formation. Dev Dyn. 221: 311–321. 2001. - PubMed
-
- Andersen TL, Hauge EM, Rolighed L, Bollerslev J, Kjærsgaard-Andersen P, and Delaisse JM. Correlation between absence of bone remodeling compartment canopies, reversal phase arrest, and deficient bone formation in post-menopausal osteoporosis. Am J Pathol. 184: 1142–1151. 2014. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
