The efficacy of aclidinium/formoterol on lung function and symptoms in patients with COPD categorized by symptom status: a pooled analysis
- PMID: 27621610
- PMCID: PMC5012634
- DOI: 10.2147/COPD.S114566
The efficacy of aclidinium/formoterol on lung function and symptoms in patients with COPD categorized by symptom status: a pooled analysis
Abstract
Background: Patients with chronic obstructive pulmonary disease (COPD) experience respiratory symptoms, which impair quality of life. This pooled analysis of two Phase III studies assessed the impact of aclidinium/formoterol on patients with COPD categorized by symptom status.
Methods: Data were pooled from two 24-week, randomized, placebo-controlled studies of twice-daily aclidinium/formoterol 400/12 µg in moderate-to-severe COPD (ACLIFORM [NCT01462942] and AUGMENT [NCT01437397]). These post hoc analyses evaluated the efficacy of aclidinium/formoterol versus placebo or monotherapies in patients defined as less/more symptomatic by a) Evaluating Respiratory Symptoms (E-RS™) score ≥10/<10 and b) Baseline Dyspnea Index score <7/≥7. Endpoints included trough and 1-hour morning postdose forced expiratory volume in 1 second (FEV1), Transition Dyspnea Index, E-RS total score, early-morning and nighttime symptom severity, early-morning limitation of activities, and exacerbation rate.
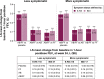
Results: Data for 3,394 patients were analyzed (mean age: 63.5 years; 60.5% male). In both definitions of less and more symptomatic patients, aclidinium/formoterol improved 1-hour morning postdose FEV1 from baseline at week 24 versus placebo (P<0.001) and both monotherapies (P<0.05). Aclidinium/formoterol improved trough FEV1 from baseline in both groups versus placebo (P<0.05) and formoterol (P<0.05); improvements were greater in more symptomatic patients. Improvements versus aclidinium were also observed in more symptomatic patients (P<0.05). Aclidinium/formoterol improved dyspnea, early-morning symptom severity, and limitation of activities versus placebo in both less and more symptomatic patients (P<0.001). In more symptomatic patients, aclidinium/formoterol also improved E-RS total score and severity of nighttime symptoms from baseline versus placebo and one or both monotherapies (P<0.05). The rate of moderate/severe exacerbations was reduced with aclidinium/formoterol versus placebo in more symptomatic patients.
Conclusion: Aclidinium/formoterol 400/12 µg provided consistent improvements in bronchodilation and symptoms versus monotherapies and reduced exacerbations versus placebo in more symptomatic patients with moderate-to-severe COPD, regardless of the definition used. Furthermore, patients with a low symptom burden achieved benefits with aclidinium/formoterol versus monotherapies in postdose FEV1, dyspnea, and early-morning symptoms.
Keywords: COPD; aclidinium; dyspnea; formoterol; lung function; symptoms.
Figures






References
-
- Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. [Accessed April 28, 2016]. [updated 2016]. Available from: http://goldcopd.org/gold-reports/ - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

