Multicomponent, peptide-targeted glycol chitosan nanoparticles containing ferrimagnetic iron oxide nanocubes for bladder cancer multimodal imaging
- PMID: 27621615
- PMCID: PMC5010162
- DOI: 10.2147/IJN.S109494
Multicomponent, peptide-targeted glycol chitosan nanoparticles containing ferrimagnetic iron oxide nanocubes for bladder cancer multimodal imaging
Abstract
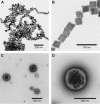
While current imaging modalities, such as magnetic resonance imaging (MRI), computed tomography, and positron emission tomography, play an important role in detecting tumors in the body, no single-modality imaging possesses all the functions needed for a complete diagnostic imaging, such as spatial resolution, signal sensitivity, and tissue penetration depth. For this reason, multimodal imaging strategies have become promising tools for advanced biomedical research and cancer diagnostics and therapeutics. In designing multimodal nanoparticles, the physicochemical properties of the nanoparticles should be engineered so that they successfully accumulate at the tumor site and minimize nonspecific uptake by other organs. Finely altering the nano-scale properties can dramatically change the biodistribution and tumor accumulation of nanoparticles in the body. In this study, we engineered multimodal nanoparticles for both MRI, by using ferrimagnetic nanocubes (NCs), and near infrared fluorescence imaging, by using cyanine 5.5 fluorescence molecules. We changed the physicochemical properties of glycol chitosan nanoparticles by conjugating bladder cancer-targeting peptides and loading many ferrimagnetic iron oxide NCs per glycol chitosan nanoparticle to improve MRI contrast. The 22 nm ferrimagnetic NCs were stabilized in physiological conditions by encapsulating them within modified chitosan nanoparticles. The multimodal nanoparticles were compared with in vivo MRI and near infrared fluorescent systems. We demonstrated significant and important changes in the biodistribution and tumor accumulation of nanoparticles with different physicochemical properties. Finally, we demonstrated that multimodal nanoparticles specifically visualize small tumors and show minimal accumulation in other organs. This work reveals the importance of finely modulating physicochemical properties in designing multimodal nanoparticles for bladder cancer imaging.
Keywords: MRI; NIRF; bladder cancer; chitosan; iron oxide; multimodal imaging.
Figures








References
-
- Xia W, Low PS. Folate-targeted therapies for cancer. J Med Chem. 2010;53(19):66–76. - PubMed
-
- Park J, Saravanakumar G, Kim K, Kwon I. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Deliv Rev. 2010;62(1):28–41. - PubMed
-
- Saravanakumar G, Min KH, Min DS, et al. Hydrotropic oligomer-conjugated glycol chitosan as a carrier of paclitaxel: synthesis, characterization, and in vivo biodistribution. J Control Release. 2009;140(3):210–217. - PubMed
-
- Min KH, Park K, Kim YS, et al. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J Control Release. 2008;127(3):208–218. - PubMed
-
- Maeda H, Matsumura Y. EPR effects. Adv Drug Deliv Rev. 2011;63(3):161–169. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

