NK Cell Influence on the Outcome of Primary Epstein-Barr Virus Infection
- PMID: 27621731
- PMCID: PMC5002423
- DOI: 10.3389/fimmu.2016.00323
NK Cell Influence on the Outcome of Primary Epstein-Barr Virus Infection
Abstract
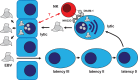
The herpesvirus Epstein-Barr virus (EBV) was discovered as the first human candidate tumor virus in Burkitt's lymphoma more than 50 years ago. Despite its strong growth transforming capacity, more than 90% of the human adult population carries this virus asymptomatically under near perfect immune control. The mode of primary EBV infection is in part responsible for EBV-associated diseases, including Hodgkin's lymphoma. It is, therefore, important to understand which circumstances lead to symptomatic primary EBV infection, called infectious mononucleosis (IM). Innate immune control of lytic viral replication by early-differentiated natural killer (NK) cells was found to attenuate IM symptoms and continuous loss of the respective NK cell subset during the first decade of life might predispose for IM during adolescence. In this review, we discuss the evidence that NK cells are involved in the immune control of EBV, mechanisms by which they might detect and control lytic EBV replication, and compare NK cell subpopulations that expand during different human herpesvirus infections.
Keywords: DNAM-1; NKG2D; humanized mice; infectious mononucleosis; lytic EBV infection.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

