Antiretroviral Therapy and Pregnancy Outcomes in Developing Countries: A Systematic Review
- PMID: 27621984
- PMCID: PMC4948169
Antiretroviral Therapy and Pregnancy Outcomes in Developing Countries: A Systematic Review
Abstract
Background: Despite significant efforts to understand adverse pregnancy outcome in women receiving Antiretroviral Therapy (ART), ART-related adverse birth outcomes are still poorly understood. We systematically review ART-related adverse birth outcomes among HIV-infected pregnant women; we also review the covariates associated with adverse birth outcomes in the aforementioned group.
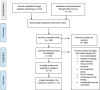
Methods: The main source for our systematic review was electronic bibliographic databases. Databases such as MEDLINE, PubMed, EMBASE and AIDSLINE were searched. Furthermore, search engines such as Google and Google Scholar were specifically searched for gray literature. Methodological quality of available literature was assessed using the Newcastle - Ottawa Quality Assessment Scale & M. Hewitt guideline. We examined a total of 1,124 papers and reviewed the studies using the PICOT criteria which stands for Patient (population), Intervention (or "Exposure"), Comparison, Outcome and Type of study. Finally, 32 methodologically fit studies were retained and included in our review.
Results: Frequently observed adverse birth outcomes included low birth weight (LBW), Preterm Birth (PB), Small for Gestational Age (SGA), while still birth and congenital anomalies were infrequent. Type of regimen such as Protease Inhibitor (PI) based regimens and timing of initiation of ART are some of the factors associated with adverse pregnancy outcomes. Covariates principally included malnutrition and other co-morbidities such as malaria and HIV.
Conclusions and public health implications: There is growing evidence in published literature suggesting that ART might be causing adverse birth outcomes among pregnant women in developing countries. There is a need to consider regimen types for HIV-infected pregnant women. There is need to design large cohort studies.
Keywords: Adverse pregnancy outcomes; Antiretroviral Therapy; Developing countries; HAART; Low birth weight; Preterm delivery; Systematic review.
Conflict of interest statement
Figures
References
-
- World Health Organization (WHO) Global HIV/AIDS response:epidemic update and health sector progress towards universal access:progress report 2014. Geneva, Switzerland: 2011.
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO). HIV in pregnancy:A Review. Switzerland, Geneva: UNAIDS; 1998. Contract Number:WHO/RHT/98.24.
-
- Segurado AC, Paiva V. Rights of HIV positive people to sexual and reproductive health Parenthood. Reproductive Health Matters. 2007;15(29):27–45. Available at http://www.rhmjournal.org.uk/ - PubMed
-
- Mock PA SN, Bhadrakom C, Siriwasin W, Chotpitayasunondh T, Chearskul S, Young NL, Roongpisuthipong A, Chinayon P, Kalish ML, Parekh B, Mastro TD. Maternal viral load and timing of mother-to-child transmission, Bangkok, Thailand. Bangkok Collaborative perinatal HIV transmission study group. AIDS. 1999;13(3):407–14. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous