Immunomodulation by MYB is associated with tumor relapse in patients with early stage colorectal cancer
- PMID: 27622014
- PMCID: PMC5006930
- DOI: 10.1080/2162402X.2016.1149667
Immunomodulation by MYB is associated with tumor relapse in patients with early stage colorectal cancer
Abstract
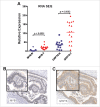
The presence of tumor immune infiltrating cells (TILs), particularly CD8(+) T-cells, is a robust predictor of outcome in patients with colorectal cancer (CRC). We revisited TIL abundance specifically in patients with microsatellite stable (MSS) CRC without evidence of lymph node or metastatic spread. Examination of the density of CD8(+) T-cells in primary tumors in the context of other pro-oncogenic markers was performed to investigate potential regulators of TILs. Two independent cohorts of patients with MSS T2-4N0M0 CRC, enriched for cases with atypical relapse, were investigated. We quantified CD8(+) and CD45RO(+) -TILs, inflammatory markers, NFkBp65, pStat3, Cyclo-oxygenase-2 (COX2) and GRP78 as well as transcription factors (TF), β-catenin and MYB. High CD8(+) TILs correlated with a better relapse-free survival in both cohorts (p = 0.002) with MYB and its target gene, GRP78 being higher in the relapse group (p = 0.001); no difference in pSTAT3 and p65 was observed. A mouse CRC (CT26) model was employed to evaluate the effect of MYB on GRP78 expression as well as T-cell infiltration. MYB over-expressing in CT26 cells increased GRP78 expression and the analysis of tumor-draining lymph nodes adjacent to tumors showed reduced T-cell activation. Furthermore, MYB over-expression reduced the efficacy of anti-PD-1 to modulate CT26 tumor growth. This high MYB and GRP78 show a reciprocal relationship with CD8(+) TILs which may be useful refining the prediction of patient outcome. These data reveal a new immunomodulatory function for MYB suggesting a basis for further development of anti-GRP78 and/or anti-MYB therapies.
Keywords: CD8+ve T Cells; GRP78; MYB; early stage colorectal cancer.
Figures





References
-
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al.. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/ 10.1126/science.1129139 - DOI - PubMed
-
- Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res 2007; 67:1883-6; PMID:17332313; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-4806 - DOI - PubMed
-
- Baxevanis CN, Papamichail M, Perez SA. Immune classification of colorectal cancer patients: impressive but how complete? Expert Opin Biol Ther 2013; 13:517-26; PMID:23289642; http://dx.doi.org/ 10.1517/14712598.2013.751971 - DOI - PubMed
-
- Ernst M, Ramsay RG. Colorectal cancer mouse models: Integrating inflammation and the stroma. J Gastroenterol Hepatol 2012; 27:39-50; PMID:22188027; http://dx.doi.org/ 10.1111/j.1440-1746.2011.06883.x - DOI - PubMed
-
- Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Eng J Med 2000; 342:69-77; PMID:10631274; http://dx.doi.org/ 10.1056/NEJM200001133420201 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
