Macrophages promote matrix protrusive and invasive function of breast cancer cells via MIP-1β dependent upregulation of MYO3A gene in breast cancer cells
- PMID: 27622050
- PMCID: PMC5006911
- DOI: 10.1080/2162402X.2016.1196299
Macrophages promote matrix protrusive and invasive function of breast cancer cells via MIP-1β dependent upregulation of MYO3A gene in breast cancer cells
Abstract
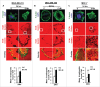
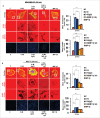
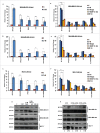
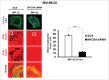
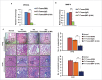
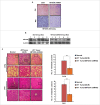
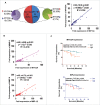
The potential of a tumor cell to metastasize profoundly depends on its microenvironment, or "niche" interactions with local components. Tumor-associated-macrophages (TAMs) are the most abundant subpopulation of tumor stroma and represent a key component of tumor microenvironment. The dynamic interaction of cancer cells with neighboring TAMs actively drive cancer progression and metastatic transformation through intercellular signaling networks that need better elucidation. Thus, current study was planned for discerning paracrine communication networks operational between TAMs, and breast cancer cells with special reference to cancer cell invasion and dissemination to distant sites. Here, we report role of MIP-1β in enhancing invasive potential of metastatic breast cancer MDA-MB-231 and MDA-MB-468 cells. In addition, the poorly metastatic MCF-7 cells were also rendered invasive by MIP-1β. The MIP-1β-driven cancer cell invasion was dependent on upregulated expression levels of MYO3A gene, which encodes an unconventional myosin super-family protein harboring a kinase domain. Ex ovo study employing Chick-embryo-model and in vivo Syngenic 4T1/BALB/c mice-model further corroborated aforementioned in vitro findings, thereby substantiating their physiological relevance. Concordantly, human breast cancer specimen exhibited significant association between mRNA expression levels of MIP-1β and MYO3A. Both, MIP-1β and MYO3A exhibited positive correlation with MMP9, an established molecular determinant of cancer cell invasion. Higher expression of these genes correlated with poor survival of breast cancer patients. Collectively, these results point toward so far undisclosed MIP-1β/MYO3A axis being operational during metastasis, wherein macrophage-derived MIP-1β potentiated cancer cell invasion and metastasis via up regulation of MYO3A gene within cancer cells. Our study exposes opportunities for devising potential anti-metastatic strategies for efficient clinical management of breast cancer.
Keywords: Invadopodia; MIP-1β; MMP-9; MYO3A; invasion; migration.
Figures





References
-
- Bendre M, Gaddy D, Nicholas RW, Suva LJ. Breast cancer metastasis to bone: it is not all about PTHrP. Clin Orthop Relat Res 2003; (415 Suppl):S39-45; PMID:14600591; http://dx.doi.org/10.1097/01.blo.0000093844.72468.f4 - DOI - PubMed
-
- Eckert MA, Yang J. Targeting invadopodia to block breast cancer metastasis. Oncotarget 2011; 2(7):562-8; PMID:21725138; http://dx.doi.org/10.18632/oncotarget.301 - DOI - PMC - PubMed
-
- Viadana E, Bross ID, Pickren JW. An autopsy study of the metastatic patterns of human leukemias. Oncology 1978; 35(2):87-96; PMID:274679; http://dx.doi.org/10.1159/000225262 - DOI - PubMed
-
- Shaffrey ME, Mut M, Asher AL, Burri SH, Chahlavi A, Chang SM, Farace E, Fiveash JB, Lang FF, Lopes MB et al.. Brain metastases. Curr Probl Surg 2004; 41(8):665-741; PMID:15354117; http://dx.doi.org/10.1067/j.cpsurg.2004.06.001 - DOI - PubMed
-
- Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 2006; 42(6):717-27; PMID:16520032; http://dx.doi.org/10.1016/j.ejca.2006.01.003 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
