Inflammatory regulatory T cells in the microenvironments of ulcerative colitis and colon carcinoma
- PMID: 27622054
- PMCID: PMC5007964
- DOI: 10.1080/2162402X.2015.1105430
Inflammatory regulatory T cells in the microenvironments of ulcerative colitis and colon carcinoma
Abstract
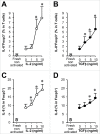
Foxp3(+)CD4(+) regulatory T (Treg) cells are thought to express negligible levels of effector cytokines, and inhibit immune responses and inflammation. Here, we have identified a population of IL-8(+)Foxp3(+)CD4(+) T cells in human peripheral blood, which is selectively increased in the microenvironments of ulcerative colitis and colon carcinoma. Phenotypically, this population is minimally overlapping with IL-17(+)Foxp3(+)CD4(+) T cells, and is different from IL-8(-)Foxp3(+)CD4(+) T cells in the same microenvironment. 40-60% of IL-8(+)Foxp3(+)CD4(+) T cells exhibit naive phenotype and express CD127, whereas IL-8(-)Foxp3(+)CD4(+) cells are basically memory T cells and express minimal CD127. The levels of CXCR5 expression are higher in IL-8(+)Foxp3(+) cells than in IL-8(-)Foxp3(+) cells. IL-2 and TGFβ induce IL-8(+)Foxp3(+) T cells. Exogenous Foxp3 expression promotes IL-8(+)Foxp3(+) T cells and inhibits effector cytokine IFNγ and IL-2 expression. Furthermore, Foxp3 binds to IL-8 proximal promoter and increases its activity. Functionally, IL-8(+)Foxp3(+) T cells inhibit T cell proliferation and effector cytokine production, but stimulate inflammatory cytokine production in the colon tissues, and promote neutrophil trafficking through IL-8. Thus, IL-8(+)Foxp3(+) cells may be an "inflammatory" Treg subset, and possess inflammatory and immunosuppressive dual biological activities. Given their dual roles and localization, these cells may be in a unique position to support tumor initiation and development in human chronic inflammatory environment.
Keywords: Colon carcinoma; IL-17; IL-8; Regulatory T cell; Th17; neutrophil; tumor immunity; ulcerative colitis.
Figures





References
-
- Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol 2002; 14:129-35; PMID:11790543; http://dx.doi.org/10.1016/S0952-7915(01)00308-9 - DOI - PubMed
-
- Wood KJ, Sakaguchi S. Regulatory Lymphocytes: Regulatory T cells in transplantation tolerance. Nat Rev Immunol 2003; 3:199-210; PMID:12658268; http://dx.doi.org/10.1038/nri1027 - DOI - PubMed
-
- Von Herrath MG, Harrison LC. Regulatory Lymphocytes: Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol 2003; 3:223-32; PMID:12658270; http://dx.doi.org/10.1038/nri1029 - DOI - PubMed
-
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2002; 2:389-400; PMID:12093005 - PubMed
-
- Francois Bach J. Regulatory lymphocytes: Regulatory T cells under scrutiny. Nat Rev Immunol 2003; 3:189-98; PMID:12658267; http://dx.doi.org/10.1038/nri1026 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
