Human CD1c(+) DCs are critical cellular mediators of immune responses induced by immunogenic cell death
- PMID: 27622063
- PMCID: PMC5007971
- DOI: 10.1080/2162402X.2016.1192739
Human CD1c(+) DCs are critical cellular mediators of immune responses induced by immunogenic cell death
Abstract
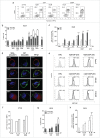
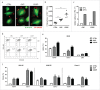
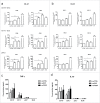
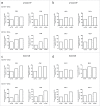
Chemotherapeutics, including the platinum compounds oxaliplatin (OXP) and cisplatin (CDDP), are standard care of treatment for cancer. Although chemotherapy has long been considered immunosuppressive, evidence now suggests that certain cytotoxic agents can efficiently stimulate antitumor responses, through the induction of a form of apoptosis, called immunogenic cell death (ICD). ICD is characterized by exposure of calreticulin and heat shock proteins (HSPs), secretion of ATP and release of high-mobility group box 1 (HMGB1). Proper activation of the immune system relies on the integration of these signals by dendritic cells (DCs). Studies on the crucial role of DCs, in the context of ICD, have been performed using mouse models or human in vitro-generated monocyte-derived DCs (moDCs), which do not fully recapitulate the in vivo situation. Here, we explore the effect of platinum-induced ICD on phenotype and function of human blood circulating DCs. Tumor cells were treated with OXP or CDDP and induction of ICD was investigated. We show that both platinum drugs triggered translocation of calreticulin and HSP70, as well as the release of ATP and HMGB1. Platinum treatment increased phagocytosis of tumor fragments by human blood DCs and enhanced phenotypic maturation of blood myeloid and plasmacytoid DCs. Moreover, upon interaction with platinum-treated tumor cells, CD1c(+) DCs efficiently stimulated allogeneic proliferation of T lymphocytes. Together, our observations indicate that platinum-treated tumor cells may exert an active stimulatory effect on human blood DCs. In particular, these data suggest that CD1c(+) DCs are critical mediators of immune responses induced by ICD.
Keywords: CD1c+ DCs; T cell proliferation; human dendritic cells; immune response; immunogenic cell death; platinum chemotherapy.
Figures


References
-
- Chabner BA, Roberts TG. Chemotherapy and the war on cancer. Nat Rev Cancer 2005; 5:65-72; PMID:15630416; http://dx.doi.org/ 10.1038/nrc1529 - DOI - PubMed
-
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008; 8:59-73; PMID:18097448; http://dx.doi.org/ 10.1038/nri2216 - DOI - PubMed
-
- Lesterhuis WJ, Haanen JBAG, Punt CJA. Cancer immunotherapy – revisited. Nat Rev Drug Discov 2011; 10:591-600; PMID:21804596; http://dx.doi.org/ 10.1038/nrd3500 - DOI - PubMed
-
- Hato SV, Khong A, de Vries IJM, Lesterhuis WJ. Molecular Pathways: The Immunogenic Effects of Platinum-Based Chemotherapeutics. Clin Cancer Res 2014; 20:2831-7; PMID:24879823; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-3141 - DOI - PubMed
-
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L et al.. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2009; 29:482-91; PMID:19881547; http://dx.doi.org/ 10.1038/onc.2009.356 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
