Retargeting cytokine-induced killer cell activity by CD16 engagement with clinical-grade antibodies
- PMID: 27622068
- PMCID: PMC5007963
- DOI: 10.1080/2162402X.2016.1199311
Retargeting cytokine-induced killer cell activity by CD16 engagement with clinical-grade antibodies
Abstract
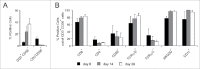
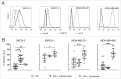
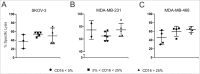
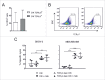
Cytokine-induced Killer (CIK) cells are a heterogeneous population of ex vivo expanded T lymphocytes capable of MHC-unrestricted antitumor activity, which share phenotypic and functional features with both NK and T cells. Preclinical data and initial clinical studies demonstrated their high tolerability in vivo, supporting CIK cells as a promising cell population for adoptive cell immunotherapy. In this study, we report for the first time that CIK cells display a donor-dependent expression of CD16, which can be engaged by trastuzumab or cetuximab to exert a potent antibody-dependent cell-mediated cytotoxicity (ADCC) against ovarian and breast cancer cell lines, leading to an increased lytic activity in vitro, and an enhanced therapeutic efficacy in vivo. Thus, an efficient tumor antigen-specific retargeting can be achieved by a combination therapy with clinical-grade monoclonal antibodies already widely used in cancer therapy, and CIK cell populations that are easily expandable in very large numbers, inexpensive, safe and do not require genetic manipulations. Overall, these data provide a new therapeutic strategy for the treatment of Her2 and EGFR expressing tumors by adoptive cell therapy, which could find wide implementation and application, and could also be expanded to the use of additional therapeutic antibodies.
Keywords: Antibody-dependent cell-mediated cytotoxicity (ADCC); cytokine induced killer (CIK) cells; immunotherapy; monoclonal antibodies.
Figures



Similar articles
-
Adoptive cell therapy of triple negative breast cancer with redirected cytokine-induced killer cells.Oncoimmunology. 2020 Jun 11;9(1):1777046. doi: 10.1080/2162402X.2020.1777046. Oncoimmunology. 2020. PMID: 32923140 Free PMC article.
-
Enhanced killing of primary ovarian cancer by retargeting autologous cytokine-induced killer cells with bispecific antibodies: a preclinical study.Clin Cancer Res. 2006 Mar 15;12(6):1859-67. doi: 10.1158/1078-0432.CCR-05-2019. Clin Cancer Res. 2006. PMID: 16551871
-
The dual-functional capability of cytokine-induced killer cells and application in tumor immunology.Hum Immunol. 2015 May;76(5):385-91. doi: 10.1016/j.humimm.2014.09.021. Epub 2014 Oct 8. Hum Immunol. 2015. PMID: 25305457 Review.
-
V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs--rituximab and trastuzumab.Int J Cancer. 2008 Jun 1;122(11):2526-34. doi: 10.1002/ijc.23365. Int J Cancer. 2008. PMID: 18307255
-
Cytokine-Induced Killer Cells As Pharmacological Tools for Cancer Immunotherapy.Front Immunol. 2017 Jul 6;8:774. doi: 10.3389/fimmu.2017.00774. eCollection 2017. Front Immunol. 2017. PMID: 28729866 Free PMC article. Review.
Cited by
-
Efficacy of cytokine-induced killer cells targeting CD40 and GITR.Oncol Lett. 2019 Feb;17(2):2425-2430. doi: 10.3892/ol.2018.9849. Epub 2018 Dec 18. Oncol Lett. 2019. PMID: 30675308 Free PMC article.
-
Immunotherapeutic Strategies for Gastric Carcinoma: A Review of Preclinical and Clinical Recent Development.Biomed Res Int. 2017;2017:5791262. doi: 10.1155/2017/5791262. Epub 2017 Jul 11. Biomed Res Int. 2017. PMID: 28781967 Free PMC article. Review.
-
Cytokine-induced killer cells: new insights for therapy of hematologic malignancies.Stem Cell Res Ther. 2024 Aug 13;15(1):254. doi: 10.1186/s13287-024-03869-z. Stem Cell Res Ther. 2024. PMID: 39135188 Free PMC article. Review.
-
CSPG4-Specific CAR.CIK Lymphocytes as a Novel Therapy for the Treatment of Multiple Soft-Tissue Sarcoma Histotypes.Clin Cancer Res. 2020 Dec 1;26(23):6321-6334. doi: 10.1158/1078-0432.CCR-20-0357. Epub 2020 Sep 8. Clin Cancer Res. 2020. PMID: 32900797 Free PMC article.
-
CD44v6 as innovative sarcoma target for CAR-redirected CIK cells.Oncoimmunology. 2018 Feb 15;7(5):e1423167. doi: 10.1080/2162402X.2017.1423167. eCollection 2018. Oncoimmunology. 2018. PMID: 29721373 Free PMC article.
References
-
- Aranda F, Buqué A, Bloy N, Castoldi F, Eggermont A, Cremer I, Fridman WH, Fucikova J, Galon J, Spisek R et al.. Trial Watch: Adoptive cell transfer for oncological indications. Oncoimmunology [Internet] 2015; 4:e1046673; PMID:26451319; http://dx.doi.org/10.1080/2162402X.2015.1046673 - DOI - PMC - PubMed
-
- Topalian SL, Drake CG, Pardoll DM. Perspective immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell [Internet] 2015; 27:450-61; PMID:25858804; http://doi.org/10.1016/j.ccell.2015.03.001 - DOI - PMC - PubMed
-
- June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: A race to the finish line. Sci Transl Med [Internet] 2015; 7:280ps7-280ps7; PMID:25810311; http://dx.doi.org/10.1126/scitranslmed.aaa3643 - DOI - PubMed
-
- Feldman SA, Assadipour Y, Kriley I, Goff SL, Rosenberg SA. Adoptive cell therapy-Tumor-Infiltrating Lymphocytes, T-Cell receptors, and chimeric antigen receptors. Semin Oncol [Internet] 2015; 42:626-39; PMID:26320066; http://dx.doi.org/10.1053/j.seminoncol.2015.05.005 - DOI - PMC - PubMed
-
- Lim O, Jung MY, Hwang YK, Shin E-C. Present and future of allogeneic natural killer cell therapy. Front Immunol [Internet] 2015; 6:286; PMID:26089823; http://dx.doi.org/10.3389/fimmu.2015.00286 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
