Electronic cigarettes for smoking cessation
- PMID: 27622384
- PMCID: PMC6457845
- DOI: 10.1002/14651858.CD010216.pub3
Electronic cigarettes for smoking cessation
Update in
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2020 Oct 14;10(10):CD010216. doi: 10.1002/14651858.CD010216.pub4. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 29;4:CD010216. doi: 10.1002/14651858.CD010216.pub5. PMID: 33052602 Free PMC article. Updated.
Abstract
Background: Electronic cigarettes (ECs) are electronic devices that heat a liquid into an aerosol for inhalation. The liquid usually comprises propylene glycol and glycerol, with or without nicotine and flavours, and stored in disposable or refillable cartridges or a reservoir. Since ECs appeared on the market in 2006 there has been a steady growth in sales. Smokers report using ECs to reduce risks of smoking, but some healthcare organizations, tobacco control advocacy groups and policy makers have been reluctant to encourage smokers to switch to ECs, citing lack of evidence of efficacy and safety. Smokers, healthcare providers and regulators are interested to know if these devices can help smokers quit and if they are safe to use for this purpose. This review is an update of a review first published in 2014.
Objectives: To evaluate the safety and effect of using ECs to help people who smoke achieve long-term smoking abstinence.
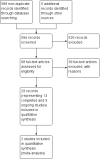
Search methods: We searched the Cochrane Tobacco Addiction Group's Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and PsycINFO for relevant records from 2004 to January 2016, together with reference checking and contact with study authors.
Selection criteria: We included randomized controlled trials (RCTs) in which current smokers (motivated or unmotivated to quit) were randomized to EC or a control condition, and which measured abstinence rates at six months or longer. As the field of EC research is new, we also included cohort follow-up studies with at least six months follow-up. We included randomized cross-over trials, RCTs and cohort follow-up studies that included at least one week of EC use for assessment of adverse events (AEs).
Data collection and analysis: We followed standard Cochrane methods for screening and data extraction. Our main outcome measure was abstinence from smoking after at least six months follow-up, and we used the most rigorous definition available (continuous, biochemically validated, longest follow-up). We used a fixed-effect Mantel-Haenszel model to calculate the risk ratio (RR) with a 95% confidence interval (CI) for each study, and where appropriate we pooled data from these studies in meta-analyses.
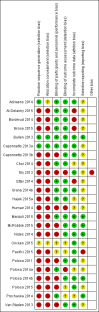
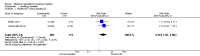
Main results: Our searches identified over 1700 records, from which we include 24 completed studies (three RCTs, two of which were eligible for our cessation meta-analysis, and 21 cohort studies). Eleven of these studies are new for this version of the review. We identified 27 ongoing studies. Two RCTs compared EC with placebo (non-nicotine) EC, with a combined sample size of 662 participants. One trial included minimal telephone support and one recruited smokers not intending to quit, and both used early EC models with low nicotine content and poor battery life. We judged the RCTs to be at low risk of bias, but under the GRADE system we rated the overall quality of the evidence for our outcomes as 'low' or 'very low', because of imprecision due to the small number of trials. A 'low' grade means that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. A 'very low' grade means we are very uncertain about the estimate. Participants using an EC were more likely to have abstained from smoking for at least six months compared with participants using placebo EC (RR 2.29, 95% CI 1.05 to 4.96; placebo 4% versus EC 9%; 2 studies; 662 participants. GRADE: low). The one study that compared EC to nicotine patch found no significant difference in six-month abstinence rates, but the confidence intervals do not rule out a clinically important difference (RR 1.26, 95% CI 0.68 to 2.34; 584 participants. GRADE: very low).Of the included studies, none reported serious adverse events considered related to EC use. The most frequently reported AEs were mouth and throat irritation, most commonly dissipating over time. One RCT provided data on the proportion of participants experiencing any adverse events. The proportion of participants in the study arms experiencing adverse events was similar (ECs vs placebo EC: RR 0.97, 95% CI 0.71 to 1.34 (298 participants); ECs vs patch: RR 0.99, 95% CI 0.81 to 1.22 (456 participants)). The second RCT reported no statistically significant difference in the frequency of AEs at three- or 12-month follow-up between the EC and placebo EC groups, and showed that in all groups the frequency of AEs (with the exception of throat irritation) decreased significantly over time.
Authors' conclusions: There is evidence from two trials that ECs help smokers to stop smoking in the long term compared with placebo ECs. However, the small number of trials, low event rates and wide confidence intervals around the estimates mean that our confidence in the result is rated 'low' by GRADE standards. The lack of difference between the effect of ECs compared with nicotine patches found in one trial is uncertain for similar reasons. None of the included studies (short- to mid-term, up to two years) detected serious adverse events considered possibly related to EC use. The most commonly reported adverse effects were irritation of the mouth and throat. The long-term safety of ECs is unknown. In this update, we found a further 15 ongoing RCTs which appear eligible for this review.
Conflict of interest statement
Within the last three years HM has received honoraria for speaking at research symposia and received benefits in kind and travel support from, and has provided consultancy to, the manufacturers of smoking cessation medications.
Within the last three years PH has provided consultancy for and received research funding from GSK, Pfizer, Novartis and other manufacturers of smoking cessation medications.
Two authors (HM, CB) have additional declarations: CB and HM were investigators on a study of ECs from an EC manufacturer (Ruyan Group, Beijing and Hong Kong). Ruyan supplied the ECs used in the trial and contracted with Health NZ Ltd. to undertake the study. Health New Zealand Ltd funded The University of Auckland to conduct the trial, independently of Ruyan Group (Holdings) Ltd. The trial design conduct, analysis and interpretation of results were conducted independently of the sponsors. CB and HM were investigators on the ASCEND EC trial funded by the Health Research Council of New Zealand that used product supplied at no charge from PGM international, a retailer of ECs.
JHB, RB and LS have no conflicts of interest to declare.
Figures


Update of
-
Electronic cigarettes for smoking cessation and reduction.Cochrane Database Syst Rev. 2014;(12):CD010216. doi: 10.1002/14651858.CD010216.pub2. Epub 2014 Dec 17. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2016 Sep 14;9:CD010216. doi: 10.1002/14651858.CD010216.pub3. PMID: 25515689 Updated.
References
References to studies included in this review
-
- Adriaens K, Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: An eight‐week Flemish study with six‐month follow‐up on smoking reduction, craving and experienced benefits and complaints. International Journal of Environmental Research and Public Health 2014;11(11):11220‐48. - PMC - PubMed
-
- Al‐Delaimy WK, Myers MG, Leas EC, Strong DR, Hofstetter CR. E‐cigarette use in the past and quitting behavior in the future: a population‐based study. American Journal of Public Health 2015;105(6):1213‐9. - PMC - PubMed
- Donzelli A. E‐cigarettes may impair ability to quit, but other explanations are possible. American Journal of Public Health 2015;105(11):e1. - PMC - PubMed
-
- Barton MK. Electronic cigarettes did not help patients with cancer stop smoking. CA: a cancer journal for clinicians 2015;65(2):85‐6. - PubMed
- Borderud SP, Li Y, Burkhalter JE, Sheffer CE, Ostroff JS. Electronic cigarette use among patients with cancer: characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer 2014;120(22):3527‐35. - PMC - PubMed
- Fillon M. Electronic cigarettes might not help cancer patients quit smoking. Journal of the National Cancer Institute 2015;107(1):496. - PubMed
-
- Brose LS, Hitchman SC, Brown J, West R, McNeill A. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1‐year follow‐up. Addiction 2015;110(7):1160‐8. - PMC - PubMed
- Brown J, West R, Beard E, Michie S, Shahab L, McNeill A. Prevalence and characteristics of e‐cigarette users in Great Britain: Findings from a general population survey of smokers. Addictive Behaviors 2014;39(6):1120‐5. - PMC - PubMed
- Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations between e‐cigarette type, frequency of use, and quitting smoking: findings from a longitudinal online panel survey in Great Britain. Nicotine & Tobacco Research 2015;17(10):1187‐94. - PMC - PubMed
-
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J. Do electronic cigarettes help smokers quit? Results from a randomised controlled trial [Abstract]. European Respiratory Society Annual Congress, 2013 September 7 ‐ 11, Barcelona, Spain 2013;42:215s‐[P1047].
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes and smoking cessation: a quandary? ‐ Authors' reply. Lancet 2014;383(9915):408‐9. - PubMed
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013;382(9905):1629‐37. - PubMed
- Bullen C, Williman J, Howe C, Laugesen M, McRobbie H, Parag V, et al. Study protocol for a randomised controlled trial of electronic cigarettes versus nicotine patch for smoking cessation. BMC Public Health 2013;13:210. - PMC - PubMed
- O'Brien B, Knight‐West O, Walker N, Parag V, Bullen C. E‐cigarettes versus NRT for smoking reduction or cessation in people with mental illness: Secondary analysis of data from the ASCEND trial. Tobacco Induced Diseases2014; Vol. 13, issue 1:5. - PMC - PubMed
References to studies excluded from this review
-
- Battista L, Iorio M, Tancredi M, Acconcia MC, Torromeo C, Barilla F, et al. Cardiovascular effects of electronic cigarettes. Circulation 2013;128:A16755.
-
- Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross‐over trial. Tobacco Control 2010;19(2):98‐103. - PubMed
References to ongoing studies
-
- Caponnetto P, Polosa R, Auditore R, Minutolo G, Signorelli M, Maglia M, et al. Smoking cessation and reduction in schizophrenia (SCARIS) with e‐cigarette: study protocol for a randomized control trial. Trials [electronic resource] 2014;15:88. - PMC - PubMed
- NCT01979796. Antismoking effects of rlectronic cigarettes in subjects with schizophrenia and their potential influence on cognitive functioning: design of a randomized trial. Smoking Cessation And Reduction In Schizophrenia (The SCARIS Study). clinicaltrials.gov/show/NCT01979796 (accessed 16 July 2014).
-
- ACTRN12612001210864. An open‐label randomised pragmatic policy trial examining effectiveness of short‐term use of Nicotine Replacement Therapy (NRT) vs short‐ or long‐term use of NRT vs short‐ or long‐term use of NRT or electronic nicotine delivery systems for smoking cessation in cigarette smokers. ACTRN12612001210864 (accessed 15 August 2016).
- Fraser D, Borland R, Gartner C. Protocol for a randomised pragmatic policy trial of nicotine products for quitting or long‐term substitution in smokers. BMC Public Health 2015;15:1026. - PMC - PubMed
-
- ISRCTN60477608. The efficacy of e‐cigarettes compared with nicotine replacement therapy, when used within the UK stop smoking service. ISRCTN60477608 2014 (accessed 15 August 2016).
-
- KCT0001277. Effect of an electronic cigarette for smoking reduction and cessation in Korean male smokers: a randomized, controlled study. KCT0001277 2014 (accessed 15 August 2016). - PubMed
-
- Lopez AA, Cobb CO, Yingst JM, Veldheer S, Hrabovsky S, Yen MS, et al. A transdisciplinary model to inform randomized clinical trial methods for electronic cigarette evaluation. BMC Public Health 2016;16(1):217. [CRS: 9400131000005107; PMCID:: PMC4778292; PUBMED: 26941050] - PMC - PubMed
- NCT02342795. Randomized controlled trial methods for novel tobacco products evaluation. clinicaltrials.gov/show/NCT02342795 (accessed 17 February 2016). [CRS: 9400131000005070]
Additional references
-
- Action on Smoking and Health. Use of electronic cigarettes (vapourisers) among adults in Great Britain. www.ash.org.uk/files/documents/ASH_891.pdf (accessed 21 July 2016).
-
- Ayers JW, Ribisl KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. American Journal of Preventive Medicine 2011;40(4):448‐53. - PubMed
-
- Balfour D. The neurobiology of tobacco dependence: A preclinical perspective on the role of dopamine projections to the nucleus. Nicotine & Tobacco Research 2004;6(6):899‐912. - PubMed
References to other published versions of this review
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

