Cell wall degradation is required for normal starch mobilisation in barley endosperm
- PMID: 27622597
- PMCID: PMC5020691
- DOI: 10.1038/srep33215
Cell wall degradation is required for normal starch mobilisation in barley endosperm
Abstract
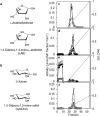
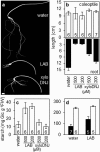
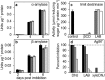
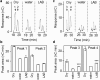
Starch degradation in barley endosperm provides carbon for early seedling growth, but the control of this process is poorly understood. We investigated whether endosperm cell wall degradation is an important determinant of the rate of starch degradation. We identified iminosugar inhibitors of enzymes that degrade the cell wall component arabinoxylan. The iminosugar 1,4-dideoxy-1, 4-imino-l-arabinitol (LAB) inhibits arabinoxylan arabinofuranohydrolase (AXAH) but does not inhibit the main starch-degrading enzymes α- and β-amylase and limit dextrinase. AXAH activity in the endosperm appears soon after the onset of germination and resides in dimers putatively containing two isoforms, AXAH1 and AXAH2. Upon grain imbibition, mobilisation of arabinoxylan and starch spreads across the endosperm from the aleurone towards the crease. The front of arabinoxylan degradation precedes that of starch degradation. Incubation of grains with LAB decreases the rate of loss of both arabinoxylan and starch, and retards the spread of both degradation processes across the endosperm. We propose that starch degradation in the endosperm is dependent on cell wall degradation, which permeabilises the walls and thus permits rapid diffusion of amylolytic enzymes. AXAH may be of particular importance in this respect. These results provide new insights into the mobilization of endosperm reserves to support early seedling growth.
Figures


References
-
- Ritchie S., Swanson S. J. & Gilroy S. From common signalling components to cell specific responses: insights from the cereal aleurone. Physiol. Plant. 15, 342–351 (2002). - PubMed
-
- Clarke S. E., Hayes P. M. & Henson C. A. Effects of single nucleotide polymorphisms in β-amylase1 alleles from barley on functional properties of the enzymes. Plant Physiol. Biochem. 41, 798–804 (2003).
-
- Muralikrishna G. & Nimala M. Cereal α-amylases - an overview. Carbohydr. Polym. 60, 163–173 (2005).
-
- Xie Z. et al.. Interactions of two abscisic-acid induced WRKY genes in repressing gibberellin signalling in aleurone cells. Plant J. 46, 231–242 (2006). - PubMed
-
- Moreno-Risueno M. A. et al.. The HvDOF19 transcription factor mediates the abscisic acid-dependent repression of hydrolase genes in germinating barley aleurone. Plant J. 51, 352–365 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/J/000C0618/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004561/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J500069/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/I017291/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

