PKCδ knockout mice are protected from para-methoxymethamphetamine-induced mitochondrial stress and associated neurotoxicity in the striatum of mice
- PMID: 27623093
- PMCID: PMC7066575
- DOI: 10.1016/j.neuint.2016.09.008
PKCδ knockout mice are protected from para-methoxymethamphetamine-induced mitochondrial stress and associated neurotoxicity in the striatum of mice
Abstract
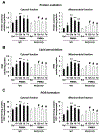
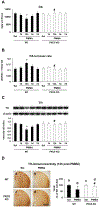
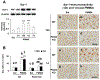
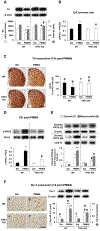
Para-methoxymethamphetamine (PMMA) is a para-ring-substituted amphetamine derivative sold worldwide as an illegal psychotropic drug. Although PMMA use has been reported to lead to severe intoxication and even death, little is known about the mechanism(s) by which PMMA exerts its neurotoxic effects. Here we found that PMMA treatment resulted in phosphorylation of protein kinase Cδ (PKCδ) and subsequent mitochondrial translocation of cleaved PKCδ. PMMA-induced oxidative stress was more pronounced in mitochondria than in the cytosol. Moreover, treatment with PMMA consistently resulted in significant reductions in mitochondrial membrane potential, mitochondrial complex I activity, and mitochondrial Mn superoxide dismutase-immunoreactivity. In contrast, PMMA treatment led to a significant increase in intramitochondrial Ca2+ level. Treatment with PMMA also significantly increased ionized calcium binding adaptor molecule 1 (Iba-1)-labeled microglial activation and upregulated tumor necrosis factor alpha (TNF-α) gene expression. PKCδ knockout attenuated these mitochondrial effects and dampened the neurotoxic effects of PMMA. Importantly, TNF-α knockout mice were significantly protected from PMMA-induced increases in phospho-PKCδ expression, mitochondrial translocation of cleaved PKCδ, and Iba-1-labeled microgliosis. Both rottlerin, a pharmacological inhibitor of PKCδ, and etanercept, a pharmacological inhibitor of TNF-α, significantly protected against PMMA-mediated induction of apoptosis, as assessed by terminal deoxynucleotidyl transferase dUDP nick end labeling (TUNEL) assays. In addition, PKCδ knockout and TNF-α knockout both resulted in decreased PMMA-mediated induction of dopaminergic loss. Therefore, our results suggest that PKCδ mediates PMMA-induced neurotoxicity by facilitating oxidative stress (mitochondria > cytosol), mitochondrial dysfunction, microglial activation, and pro-apoptotic signaling. Our results also indicate that PMMA-induced PKCδ activation requires the proinflammatory cytokine TNF-α.
Keywords: Microglia; Mitochondria; Oxidative stress; Para-methoxymethamphetamine; Protein kinase Cδ; Tumor necrosis factor-α.
Copyright © 2016. Published by Elsevier Ltd.
Conflict of interest statement
Conflict of interest disclosure
There is no conflict of interest
Figures



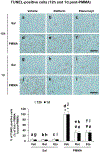
References
-
- Bach MV, Coutts RT, Baker GB, 1999. Involvement of CYP2D6 in the in vitro metabolism of amphetamine, two N-alkylamphetamines and their 4-methoxylated derivatives. Xenobiotica 29, 719–732. - PubMed
-
- Bankson MG, Cunningham KA, 2002. Pharmacological studies of the acute effects of (+)-3,4-methylenedioxymethamphetamine on locomotor activity: role of 5-HT(1B/1D) and 5-HT(2) receptors. Neuropsychopharmacology 26, 40–52. - PubMed
-
- Block ML, Hong JS, 2005. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog. Neurobiol 76, 77–98. - PubMed
-
- Block ML, Zecca L, Hong JS, 2007. Microglia-mediated neurotoxcity: uncovering the molecular mechanisms. Nat. Rev. Neurosci 8, 57–69. - PubMed
-
- Bubar MJ, Pack KM, Frankel PS, Cunningham KA, 2004. Effects of dopamine D1- or D2-like receptor antagonists on the hypermotive and discriminative stimulus effects of (+)-MDMA. Psychopharmacology (Berl) 173, 326–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

