Biomarker analyses in REGARD gastric/GEJ carcinoma patients treated with VEGFR2-targeted antibody ramucirumab
- PMID: 27623234
- PMCID: PMC5061911
- DOI: 10.1038/bjc.2016.293
Biomarker analyses in REGARD gastric/GEJ carcinoma patients treated with VEGFR2-targeted antibody ramucirumab
Abstract
Background: Angiogenesis inhibition is an important strategy for cancer treatment. Ramucirumab, a human IgG1 monoclonal antibody that targets VEGF receptor 2 (VEGFR2), inhibits VEGF-A, -C, -D binding and endothelial cell proliferation. To attempt to identify prognostic and predictive biomarkers, retrospective analyses were used to assess tumour (HER2, VEGFR2) and serum (VEGF-C and -D, and soluble (s) VEGFR1 and 3) biomarkers in phase 3 REGARD patients with metastatic gastric/gastroesophageal junction carcinoma.
Methods: A total of 152 out of 355 (43%) patients randomised to ramucirumab or placebo had ⩾1 evaluable biomarker result using VEGFR2 immunohistochemistry or HER2, immunohistochemistry or FISH, of blinded baseline tumour tissue samples. Serum samples (32 patients, 9%) were assayed for VEGF-C and -D, and sVEGFR1 and 3.
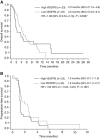
Results: None of the biomarkers tested were associated with ramucirumab efficacy at a level of statistical significance. High VEGFR2 endothelial expression was associated with a non-significant prognostic trend toward shorter progression-free survival (high vs low HR=1.65, 95% CI=0.84,3.23). Treatment with ramucirumab was associated with a trend toward improved survival in both high (HR=0.69, 95% CI=0.38, 1.22) and low (HR=0.73, 95% CI=0.42, 1.26) VEGFR2 subgroups. The benefit associated with ramucirumab did not appear to differ by tumoural HER2 expression.
Conclusions: REGARD exploratory analyses did not identify a strong potentially predictive biomarker of ramucirumab efficacy; however, statistical power was limited.
Conflict of interest statement
IC, BM, MAT, JT report speaker honoraria from Lilly and non-Lilly pharmaceutical companies. JAA, IC report a commercial research grant. IC, CSF, JTa, MAT, JTo report serving on the Advisory Board for Eli Lilly and/or other companies. CWC, ME, DF, RRH, SAM are employees of Eli Lilly and Company. The remaining authors declare no conflict of interest.
Figures



References
-
- Bais C, Rabe C, Wild N (2014) Comprehensive reassessment of plasma VEGFA (pVEGFA) as a candidate predictive biomarker for bevacizumab (Bv) in 13 pivotal trials (seven indications). J Clin Oncol 32: 5s.
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376: 687–697. - PubMed
-
- Betts G, Valentine H, Pritchard S, Swindell R, Williams V, Morgan S, Griffiths EA, Welch I, West C, Womack C (2014) FGFR2, HER2 and cMet in gastric adenocarcinoma: detection, prognostic significance and assessment of downstream pathway activation. Virchows Arch 464: 145–156. - PubMed
-
- Cao W, Fan R, Yang W, Wu Y (2014) VEGF-C expression is associated with the poor survival in gastric cancer tissue. Tumour Biol 35: 3377–3383. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

