Down-Regulation of CD62L Shedding in T Cells by CD39+ Regulatory T Cells Leads to Defective Sensitization in Contact Hypersensitivity Reactions
- PMID: 27623510
- PMCID: PMC5472987
- DOI: 10.1016/j.jid.2016.08.023
Down-Regulation of CD62L Shedding in T Cells by CD39+ Regulatory T Cells Leads to Defective Sensitization in Contact Hypersensitivity Reactions
Abstract
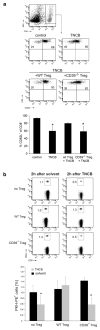
Injection of regulatory T cells (Tregs) followed by sensitization with 2,4,6-trinitrochlorobenzene induced a transient increase in size and cellularity of skin-draining lymph nodes (LNs) in mice. This led us to hypothesize that Tregs may affect the trafficking of T cells from and to peripheral LNs. Two to three hours after sensitization, we found fewer CD8+ T cells expressing CD62L in LNs compared with untreated controls. Injection of wild-type Tregs prevented this down-regulation of CD62L. In contrast, Tregs devoid of the adenosine triphosphate (ATP)-degrading ecto-enzyme CD39 were unable to do so. As for the mechanism of CD62L regulation, we found that ATP, which is released in skin upon hapten-exposure, is inducing the protease ADAM17 in LN-residing T cells via engagement of P2X7 ATP receptors. ADAM17 cleaves CD62L from the surface of CD8+ T cells, which in turn provide a signal for T cells to leave the LNs. This regulation of CD62L is disturbed by the presence of Tregs, because Tregs remove extracellular ATP from the tissue by activity of CD39 and, therefore, abrogate the shedding of CD62L. Thus, these data indicate that the regulation of ATP turnover by Tregs in skin and LNs is an important modulator for immune responses.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors state no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

