Transcranial doppler re-screening of subjects who participated in STOP and STOP II
- PMID: 27623561
- PMCID: PMC5155448
- DOI: 10.1002/ajh.24551
Transcranial doppler re-screening of subjects who participated in STOP and STOP II
Abstract
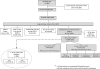
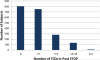
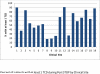
In children with Sickle Cell Disease, the combination of risk stratification with Transcranial Doppler Ultrasound (TCD) and selective chronic red cell transfusion (CRCT-the STOP Protocol) is one of the most effective stroke prevention strategies in medicine. How fully it is being implemented is unclear. Nineteen of 26 sites that conducted the two pivotal clinical trials (STOP and STOP II) participated in Post STOP, a comprehensive medical records review assessing protocol implementation in the 10-15 years since the trials ended. Professional abstractors identified medical records in the Post STOP era in 2851 (74%) of the 3,840 children who took part in STOP and/or STOP II, and documented TCD rescreening, maintenance of CRCT in those at risk, and stroke. Among 1,896 children eligible for TCD rescreening (target group), evidence of any rescreening was found in 1,090 (57%). There was wide site variation in TCD rescreening ranging from 18% to 91% of eligible children. Both younger age and having a conditional TCD during STOP/II were associated with a higher likelihood of having a TCD in Post STOP. Sixty eight new abnormal, high risk cases were identified. Despite clear evidence of benefit the STOP protocol is not fully implemented even at experienced sites. Site variation suggests that system improvements might remove barriers to implementation and result in even greater reduction of ischemic stroke in children with SCD. Am. J. Hematol. 91:1191-1194, 2016. © 2016 Wiley Periodicals, Inc.
© 2016 Wiley Periodicals, Inc.
Figures
Comment in
-
A comment on improving transcranial Doppler ultrasonography screening in children with sickle cell anemia.Am J Hematol. 2017 Jun;92(6):E121-E122. doi: 10.1002/ajh.24727. Epub 2017 Apr 29. Am J Hematol. 2017. PMID: 28335074 No abstract available.
References
-
- Adams R, McKie V, Nichols F, et al. The Use of Transcranial Ultrasonography to Predict Stroke in Sickle Cell Disease. New England Journal of Medicine. 1992;326(9):605–610. - PubMed
-
- Adams R, McKie V, Hsu L, et al. Prevention of a First Stroke by Transfusions in Children with Sickle Cell Anemia and Abnormal Results on Transcranial Doppler Ultrasonography. New England Journal of Medicine. 1998;339(1):5–11. - PubMed
-
- Adams R, Brambilla D, for the STOP II Consortium Discontinuing Prophylactic Transfusions Used to Prevent Stroke in Sickle Cell Disease. New England Journal of Medicine. 2005;353(26):2769–2778. - PubMed
-
- Adams RJ, McKie VC, Brambilla DJ, et al. Stroke Prevention Trial in Sickle Cell Anemia (“STOP”): Study Design. Controlled Clinical Trials. 1997;19:110–129. - PubMed
-
- McCarville M, Goodin G, Fortner G, et al. Evaluation of a comprehensive transcranial doppler screening program for children with sickle cell anemia. Pediatric Blood Cancer. 2008;50(4):818–821. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical