Chloroquine Suppresses the Development of Hypertension in Spontaneously Hypertensive Rats
- PMID: 27623761
- PMCID: PMC5225945
- DOI: 10.1093/ajh/hpw113
Chloroquine Suppresses the Development of Hypertension in Spontaneously Hypertensive Rats
Abstract
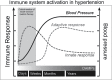
Background: Innate immune system responses to damage-associated molecular patterns (DAMPs) are involved in hypertension. However, the mechanisms of this contribution are not well understood. Circulating mitochondrial DNA is a DAMP that activates Toll-like receptor (TLR) 9 and is elevated in spontaneously hypertensive rats (SHR). Therefore, we hypothesized that lysosomotropic agent chloroquine (CQ) would impair TLR9 signaling, as well as prevent the development of hypertension and immune cell recruitment to the vasculature, in SHR.
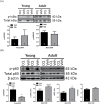
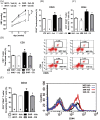
Methods: Initially, adult SHR and Wistar-Kyoto (WKY) rats (12 weeks old), as well as a group of young SHR (5 weeks old), were treated with CQ (40mg/kg/day) or vehicle (saline) via intraperitoneal injections for 21 days and then TLR9-myeloid differentiation primary response protein (MyD88) signaling proteins were assessed in mesenteric resistance arteries (MRA) via western blot. Subsequently, young SHR and WKY were treated from 5-8 weeks of age and then were allowed to mature without further treatment. Blood pressure was measured pretreatment, posttreatment, and after maturation, and immune cell recruitment to the vasculature was measured via flow cytometry after maturation.
Results: In MRA from adult SHR, CQ increased the expression of MyD88-dependent proteins, whereas young SHR MRA exhibited a decrease. This inhibition was subsequently associated with suppression of blood pressure, as well as decreased counts of circulating T cells and vascular infiltrating leukocytes in SHR, when CQ was administered during the prehypertensive phase.
Conclusions: These data bring into question the participation of TLRs during the maintenance phase of hypertension and promote the exploration of innate immune system therapy during the critical developmental phase.
Keywords: Toll-like receptors.; blood pressure; hypertension; immune system.
© American Journal of Hypertension, Ltd 2016. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Toll-Like Receptors, Hypertension, and an Antimalarial Drug.Am J Hypertens. 2017 Feb;30(2):118-119. doi: 10.1093/ajh/hpw128. Epub 2016 Oct 4. Am J Hypertens. 2017. PMID: 27702749 Free PMC article. No abstract available.
References
-
- Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med 1967; 25:257–264. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

