Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis
- PMID: 27623861
- PMCID: PMC5022202
- DOI: 10.1186/s12916-016-0673-8
Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis
Abstract
Background: To perform a systematic review and network meta-analysis (NMA) to compare the risk of serious infections with immunosuppressive medications and glucocorticoids in lupus nephritis.
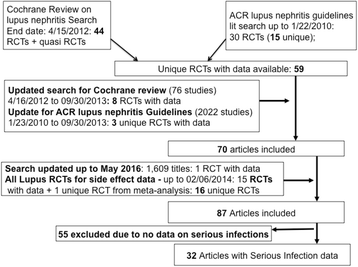
Methods: A trained librarian performed two searches: (1) PubMed for all lupus nephritis trials from the end dates for the systematic review for the 2012 American College of Rheumatology (ACR) lupus nephritis treatment guidelines and the 2012 Cochrane Systematic Review on treatments for lupus nephritis, to September 2013; and (2) PubMed and SCOPUS for all lupus trials (excluding lupus nephritis) from inception to February 2014, to obtain additional trials for harms data in any lupus patient. The search was updated to May 2016. Duplicate title/abstract review and duplicate data abstractions by two abstractors independently was performed for all eligible studies, including those studies abstracted for the 2012 ACR lupus nephritis treatment guidelines and the 2012 Cochrane Systematic Review on lupus nephritis treatments. We performed a systematic review and a Bayesian NMA, including randomized controlled trials (RCTs) of immunosuppressive drugs or glucocorticoids in patients with lupus nephritis assessing serious infection risk. Markov chain Monte Carlo methods were used to model 95 % credible intervals (CrI). Sensitivity analyses examined the robustness of estimates.
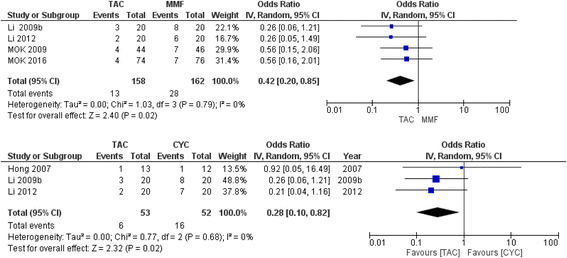
Results: A total of 32 RCTs with 2611 patients provided data. There were 26 two-arm, five three-arm, and one four-arm trials. We found that tacrolimus was associated with significantly lower risk of serious infections compared to glucocorticoids, cyclophosphamide (CYC), mycophenolate mofetil (MMF), and azathioprine (AZA) with odds ratios (95 % CrI) of 0.33 (0.12-0.88), 0.37 (0.15-0.87), 0.340 (0.18-0.81), and 0.32 (0.12-0.81), respectively. Conversely, CYC low dose (LD), CYC high dose (HD), and HD glucocorticoids were associated with higher odds of serious infections compared to tacrolimus, ranging from 4.84 to 12.83. We also found that MMF followed by AZA (MMF-AZA) was associated with significantly lower risk of serious infections as compared to CYC LD, CYC HD, CYC-AZA, or HD glucocorticoids with odds ratios (95 % CrI) of 0.09 (0.01-0.76), 0.07 (0.01-0.54), 0.14 (0.02-0.71), and 0.03 (0.00-0.56), respectively. Estimates were similar to pair-wise meta-analyses. Sensitivity analyses that varied estimate (odds ratio vs. Peto's odds ratio), method (random vs. fixed effects model), data (sepsis vs. serious infection data; exclusion of observational studies), treatment grouping (CYC and CYC HD as a combined treatment group vs. separate), made little/no difference to these estimates.
Conclusions: Tacrolimus and MMF-AZA combination were associated with lower risk of serious infections compared to other immunosuppressive drugs or glucocorticoids for lupus nephritis. In conjunction with comparative efficacy data, these data can help patients make informed decisions about treatment options for lupus nephritis.
Prospero registration: CRD42016032965.
Keywords: Cyclophosphamide; Glucocorticoids; Immunosuppressive drugs; Lupus; Lupus nephritis; Mycophenolate mofetil; Network meta-analysis; Serious infections; Tacrolimus.
Figures


References
-
- Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejia JC, Aydintug AO, Chwalinska-Sadowska H, de Ramon E, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine. 2003;82(5):299–308. doi: 10.1097/01.md.0000091181.93122.55. - DOI - PubMed
-
- Garcia Popa-Lisseanu MG, Greisinger A, Richardson M, O’Malley KJ, Janssen NM, Marcus DM, Tagore J, Suarez-Almazor ME. Determinants of treatment adherence in ethnically diverse, economically disadvantaged patients with rheumatic disease. J Rheumatol. 2005;32(5):913–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

