Biophysical Characterization of a Thermoalkaliphilic Molecular Motor with a High Stepping Torque Gives Insight into Evolutionary ATP Synthase Adaptation
- PMID: 27624936
- PMCID: PMC5104922
- DOI: 10.1074/jbc.M116.743633
Biophysical Characterization of a Thermoalkaliphilic Molecular Motor with a High Stepping Torque Gives Insight into Evolutionary ATP Synthase Adaptation
Abstract
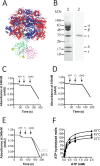
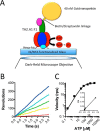
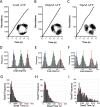
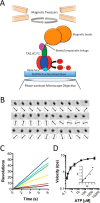
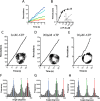
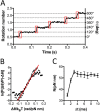
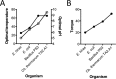
F1F0 ATP synthases are bidirectional molecular motors that translocate protons across the cell membrane by either synthesizing or hydrolyzing ATP. Alkaliphile ATP synthases are highly adapted, performing oxidative phosphorylation at high pH against an inverted pH gradient (acidin/alkalineout). Unlike mesophilic ATP synthases, alkaliphilic enzymes have tightly regulated ATP hydrolysis activity, which can be relieved in the presence of lauryldimethylamine oxide. Here, we characterized the rotary dynamics of the Caldalkalibacillus thermarum TA2.A1 F1 ATPase (TA2F1) with two forms of single molecule analysis, a magnetic bead duplex and a gold nanoparticle. TA2F1 rotated in a counterclockwise direction in both systems, adhering to Michaelis-Menten kinetics with a maximum rotation rate (Vmax) of 112.4 revolutions/s. TA2F1 displayed 120° unitary steps coupled with ATP hydrolysis. Torque measurements revealed the highest torque (52.4 piconewtons) derived from an F1 molecule using fluctuation theorem. The implications of high torque in terms of extreme environment adaptation are discussed.
Keywords: ATP synthase; F1F0-ATPase; adaptation; enzyme kinetics; enzyme mechanism; respiratory chain; rotation; single molecule biophysics; thermoalkaliphile; torque.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Yoshida M., Muneyuki E., and Hisabori T. (2001) ATP synthase—a marvellous rotary engine of the cell. Nat. Rev. Mol. Cell Biol. 2, 669–677 - PubMed
-
- Abrahams J. P., Leslie A. G., Lutter R., and Walker J. E. (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370, 621–628 - PubMed
-
- Weber J., and Senior A. E. (1997) Catalytic mechanism of F1-ATPase. Biochim. Biophys. Acta 1319, 19–58 - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

