Novel Insights into the PKCβ-dependent Regulation of the Oxidoreductase p66Shc
- PMID: 27624939
- PMCID: PMC5095410
- DOI: 10.1074/jbc.M116.752766
Novel Insights into the PKCβ-dependent Regulation of the Oxidoreductase p66Shc
Abstract
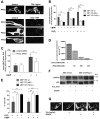
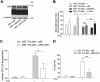
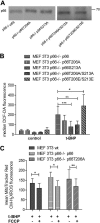
Dysfunctional mitochondria contribute to the development of many diseases and pathological conditions through the excessive production of reactive oxygen species (ROS), and, where studied, ablation of p66Shc (p66) was beneficial. p66 translocates to the mitochondria and oxidizes cytochrome c to yield H2O2, which in turn initiates cell death. PKCβ-mediated phosphorylation of serine 36 in p66 has been implicated as a key regulatory step preceding mitochondrial translocation, ROS production, and cell death, and PKCβ thus may provide a target for therapeutic intervention. We performed a reassessment of PKCβ regulation of the oxidoreductase activity of p66. Although our experiments did not substantiate Ser36 phosphorylation by PKCβ, they instead provided evidence for Ser139 and Ser213 as PKCβ phosphorylation sites regulating the pro-oxidant and pro-apoptotic function of p66. Mutation of another predicted PKCβ phosphorylation site also located in the phosphotyrosine binding domain, threonine 206, had no phenotype. Intriguingly, p66 with Thr206 and Ser213 mutated to glutamic acid showed a gain-of-function phenotype with significantly increased ROS production and cell death induction. Taken together, these data argue for a complex mechanism of PKCβ-dependent regulation of p66 activation involving Ser139 and a motif surrounding Ser213.
Keywords: PKC; cell death; p66shc; phosphorylation; phosphotyrosine binding (PTB) domain; reactive oxygen species (ROS); redox signaling.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Kulkarni A. C., Kuppusamy P., and Parinandi N. (2007) Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid. Redox Signal. 9, 1717–1730 - PubMed
-
- McKay A., Cassidy D., Sutherland F., and Dixon E. (2008) Clinical results of N-acetylcysteine after major hepatic surgery: a review. J. Hepatobiliary Pancreat. Surg. 15, 473–478 - PubMed
-
- Fuller T. F., Serkova N., Niemann C. U., and Freise C. E. (2004) Influence of donor pretreatment with N-acetylcysteine on ischemia/reperfusion injury in rat kidney grafts. J. Urol. 171, 1296–1300 - PubMed
-
- Kozower B. D., Christofidou-Solomidou M., Sweitzer T. D., Muro S., Buerk D. G., Solomides C. C., Albelda S. M., Patterson G. A., and Muzykantov V. R. (2003) Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 21, 392–398 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

