Chemical proteomic map of dimethyl fumarate-sensitive cysteines in primary human T cells
- PMID: 27625306
- PMCID: PMC5068918
- DOI: 10.1126/scisignal.aaf7694
Chemical proteomic map of dimethyl fumarate-sensitive cysteines in primary human T cells
Abstract
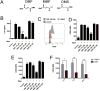
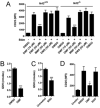
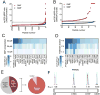
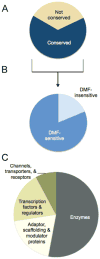
Dimethyl fumarate (DMF) is an electrophilic drug that is used to treat autoimmune conditions, including multiple sclerosis and psoriasis. The mechanism of action of DMF is unclear but may involve the covalent modification of proteins or DMF serving as a prodrug that is converted to monomethyl fumarate (MMF). We found that DMF, but not MMF, blocked the activation of primary human and mouse T cells. Using a quantitative, site-specific chemical proteomic platform, we determined the DMF sensitivity of >2400 cysteine residues in human T cells. Cysteines sensitive to DMF, but not MMF, were identified in several proteins with established biochemical or genetic links to T cell function, including protein kinase Cθ (PKCθ). DMF blocked the association of PKCθ with the costimulatory receptor CD28 by perturbing a CXXC motif in the C2 domain of this kinase. Mutation of these DMF-sensitive cysteines also impaired PKCθ-CD28 interactions and T cell activation, designating the C2 domain of PKCθ as a key functional, electrophile-sensing module important for T cell biology.
Copyright © 2016, American Association for the Advancement of Science.
Figures
Similar articles
-
Dimethyl Fumarate Disrupts Human Innate Immune Signaling by Targeting the IRAK4-MyD88 Complex.J Immunol. 2019 May 1;202(9):2737-2746. doi: 10.4049/jimmunol.1801627. Epub 2019 Mar 18. J Immunol. 2019. PMID: 30885957 Free PMC article.
-
Dimethyl fumarate is an allosteric covalent inhibitor of the p90 ribosomal S6 kinases.Nat Commun. 2018 Oct 19;9(1):4344. doi: 10.1038/s41467-018-06787-w. Nat Commun. 2018. PMID: 30341347 Free PMC article.
-
Analyzing Cysteine Site Neighbors in Proteins to Reveal Dimethyl Fumarate Targets.Proteomics. 2019 Feb;19(4):e1800301. doi: 10.1002/pmic.201800301. Epub 2019 Jan 25. Proteomics. 2019. PMID: 30633445
-
Dimethyl fumarate downregulates the immune response through the HCA2/GPR109A pathway: Implications for the treatment of multiple sclerosis.Mult Scler Relat Disord. 2018 Jul;23:46-50. doi: 10.1016/j.msard.2018.04.016. Epub 2018 Apr 25. Mult Scler Relat Disord. 2018. PMID: 29763776 Review.
-
Utilization of Dimethyl Fumarate and Related Molecules for Treatment of Multiple Sclerosis, Cancer, and Other Diseases.Front Immunol. 2016 Jul 22;7:278. doi: 10.3389/fimmu.2016.00278. eCollection 2016. Front Immunol. 2016. PMID: 27499754 Free PMC article. Review.
Cited by
-
Dimethyl Fumarate Induces Metabolic Crisie to Suppress Pancreatic Carcinoma.Front Pharmacol. 2021 Feb 22;12:617714. doi: 10.3389/fphar.2021.617714. eCollection 2021. Front Pharmacol. 2021. PMID: 33692690 Free PMC article.
-
Repurposing of Drugs Targeting YAP-TEAD Functions.Cancers (Basel). 2018 Sep 14;10(9):329. doi: 10.3390/cancers10090329. Cancers (Basel). 2018. PMID: 30223434 Free PMC article. Review.
-
Proteomics and Beyond: Cell Decision-Making Shaped by Reactive Electrophiles.Trends Biochem Sci. 2019 Jan;44(1):75-89. doi: 10.1016/j.tibs.2018.09.014. Epub 2018 Oct 13. Trends Biochem Sci. 2019. PMID: 30327250 Free PMC article. Review.
-
Dimethyl Fumarate Disrupts Human Innate Immune Signaling by Targeting the IRAK4-MyD88 Complex.J Immunol. 2019 May 1;202(9):2737-2746. doi: 10.4049/jimmunol.1801627. Epub 2019 Mar 18. J Immunol. 2019. PMID: 30885957 Free PMC article.
-
Ube2V2 Is a Rosetta Stone Bridging Redox and Ubiquitin Codes, Coordinating DNA Damage Responses.ACS Cent Sci. 2018 Feb 28;4(2):246-259. doi: 10.1021/acscentsci.7b00556. Epub 2018 Jan 17. ACS Cent Sci. 2018. PMID: 29532025 Free PMC article.
References
-
- Ropper AH. The “;poison chair”; treatment for multiple sclerosis. The New England journal of medicine. 2012;367:1149–1150. - PubMed
-
- Schimrigk S, Brune N, Hellwig K, Lukas C, Bellenberg B, Rieks M, Hoffmann V, Pohlau D, Przuntek H. Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. European journal of neurology. 2006;13:604–610. - PubMed
-
- Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT, Investigators DS. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. The New England journal of medicine. 2012;367:1098–1107. - PubMed
-
- Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, Yang M, Raghupathi K, Novas M, Sweetser MT, Viglietta V, Dawson KT, Investigators CS. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. The New England journal of medicine. 2012;367:1087–1097. - PubMed
-
- Linker RA, Gold R. Dimethyl fumarate for treatment of multiple sclerosis: mechanism of action, effectiveness, and side effects. Current neurology and neuroscience reports. 2013;13:394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

