Intranasal Opioid Administration in Rhesus Monkeys: PET Imaging and Antinociception
- PMID: 27625351
- PMCID: PMC5074481
- DOI: 10.1124/jpet.116.235192
Intranasal Opioid Administration in Rhesus Monkeys: PET Imaging and Antinociception
Abstract
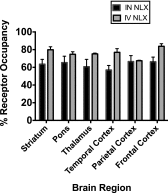
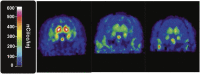
The goal of this study was to evaluate the effects of intranasally administered opioids in rhesus monkeys using the tail-withdrawal assay, and to correlate these effects with measures of receptor occupancy using positron emission tomography (PET) imaging. Initial experiments characterized the antinociceptive effects of intranasal (IN) fentanyl and buprenorphine relative to intramuscular (IM) injection. Fentanyl (0.010-0.032 mg/kg) and buprenorphine (0.1-1.0 mg/kg) produced dose-dependent increases in tail-withdrawal latency that did not differ between routes of delivery. The second experiment compared the ability of IN and intravenous (IV) naloxone (NLX) to block the antinociceptive effects IV fentanyl, and to measure receptor occupancy at equipotent doses of NLX using PET imaging. IN and IV NLX (0.0032-0.032 mg/kg) produced dose-dependent decreases in fentanyl-induced antinociception. Again, there was no difference observed in overall potency between routes. PET imaging showed that IV and IN NLX produced similar decreases in receptor occupancy as measured by [11C]carfentanil blocking, although there was a trend for IV NLX to produce marginally greater occupancy changes. This study validated the first procedures to evaluate the IN effects of opioids in rhesus monkeys.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures




References
-
- Barton ED, Colwell CB, Wolfe T, Fosnocht D, Gravitz C, Bryan T, Dunn W, Benson J, Bailey J. (2005) Efficacy of intranasal naloxone as a needleless alternative for treatment of opioid overdose in the prehospital setting. J Emerg Med 29:265–271. - PubMed
-
- Butelman ER, France CP, Woods JH. (1993) Apparent pA2 analysis on the respiratory depressant effects of alfentanil, etonitazene, ethylketocyclazocine (EKC) and Mr2033 in rhesus monkeys. J Pharmacol Exp Ther 264:145–151. - PubMed
-
- Cheng T, Zhao Y, Li X, Lin F, Xu Y, Zhang X, Li Y, Wang R, Lai L. (2007) Computation of octanol-water partition coefficients by guiding an additive model with knowledge. J Chem Inf Model 47:2140–2148. - PubMed
-
- Christrup LL, Foster D, Popper LD, Troen T, Upton R. (2008) Pharmacokinetics, efficacy, and tolerability of fentanyl following intranasal versus intravenous administration in adults undergoing third-molar extraction: a randomized, double-blind, double-dummy, two-way, crossover study. Clin Ther 30:469–481. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

