A trial of unrelated donor marrow transplantation for children with severe sickle cell disease
- PMID: 27625358
- PMCID: PMC5123194
- DOI: 10.1182/blood-2016-05-715870
A trial of unrelated donor marrow transplantation for children with severe sickle cell disease
Abstract
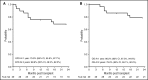
Children with sickle cell disease experience organ damage, impaired quality of life, and premature mortality. Allogeneic bone marrow transplant from an HLA-matched sibling can halt disease progression but is limited by donor availability. A Blood and Marrow Transplant Clinical Trials Network (BMT CTN) phase 2 trial conducted from 2008 to 2014 enrolled 30 children aged 4 to 19 years; 29 were eligible for evaluation. The primary objective was 1-year event-free survival (EFS) after HLA allele-matched (at HLA-A, -B, -C, and -DRB1 loci) unrelated donor transplant. The conditioning regimen included alemtuzumab, fludarabine, and melphalan. Graft-versus-host disease (GVHD) prophylaxis included calcineurin inhibitor, short-course methotrexate, and methylprednisolone. Transplant indications included stroke (n = 12), transcranial Doppler velocity >200 cm/s (n = 2), ≥3 vaso-occlusive pain crises per year (n = 12), or ≥2 acute chest syndrome episodes (n = 4) in the 2 years preceding enrollment. Median follow-up was 26 months (range, 12-62 months); graft rejection was 10%. The 1- and 2-year EFS rates were 76% and 69%, respectively. The corresponding rates for overall survival were 86% and 79%. The day 100 incidence rate of grade II-IV acute GVHD was 28%, and the 1-year incidence rate of chronic GVHD was 62%; 38% classified as extensive. There were 7 GVHD-related deaths. A 34% incidence of posterior reversible encephalopathy syndrome was noted in the first 6 months. Although the 1-year EFS met the prespecified target of ≥75%, this regimen cannot be considered sufficiently safe for widespread adoption without modifications to achieve more effective GVHD prophylaxis. The BMT CTN #0601 trial was registered at www.clinicaltrials.gov as #NCT00745420.
Figures
Comment in
-
Sickle cell disease: the price of cure.Blood. 2016 Nov 24;128(21):2486-2488. doi: 10.1182/blood-2016-10-740969. Blood. 2016. PMID: 27884835 No abstract available.
References
-
- Walters MC, Patience M, Leisenring W, et al. . Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335(6):369-376. - PubMed
-
- Panepinto JA, Walters MC, Carreras J, et al. ; Non-Malignant Marrow Disorders Working Committee, Center for International Blood and Marrow Transplant Research. Matched-related donor transplantation for sickle cell disease: report from the Center for International Blood and Transplant Research. Br J Haematol. 2007;137(5):479-485. - PubMed
-
- Locatelli F, Kabbara N, Ruggeri A, et al. ; Eurocord and European Blood and Marrow Transplantation (EBMT) group. Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood. 2013;122(6):1072-1078. - PubMed
-
- Bernaudin F, Socie G, Kuentz M, et al. ; SFGM-TC. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110(7):2749-2756. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous