Causal Interrogation of Neuronal Networks and Behavior through Virally Transduced Ivermectin Receptors
- PMID: 27625595
- PMCID: PMC5004486
- DOI: 10.3389/fnmol.2016.00075
Causal Interrogation of Neuronal Networks and Behavior through Virally Transduced Ivermectin Receptors
Abstract
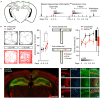
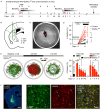
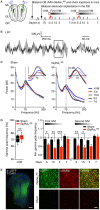
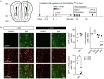
The causal interrogation of neuronal networks involved in specific behaviors requires the spatially and temporally controlled modulation of neuronal activity. For long-term manipulation of neuronal activity, chemogenetic tools provide a reasonable alternative to short-term optogenetic approaches. Here we show that virus mediated gene transfer of the ivermectin (IVM) activated glycine receptor mutant GlyRα1 (AG) can be used for the selective and reversible silencing of specific neuronal networks in mice. In the striatum, dorsal hippocampus, and olfactory bulb, GlyRα1 (AG) promoted IVM dependent effects in representative behavioral assays. Moreover, GlyRα1 (AG) mediated silencing had a strong and reversible impact on neuronal ensemble activity and c-Fos activation in the olfactory bulb. Together our results demonstrate that long-term, reversible and re-inducible neuronal silencing via GlyRα1 (AG) is a promising tool for the interrogation of network mechanisms underlying the control of behavior and memory formation.
Keywords: glycine receptor; ivermectin; neuronal silencing; odor discrimination; rAAV.
Figures


Similar articles
-
Ivermectin-Activated, Cation-Permeable Glycine Receptors for the Chemogenetic Control of Neuronal Excitation.ACS Chem Neurosci. 2016 Dec 21;7(12):1647-1657. doi: 10.1021/acschemneuro.6b00168. Epub 2016 Sep 27. ACS Chem Neurosci. 2016. PMID: 27611437
-
Haploinsufficiency of autism causative gene Tbr1 impairs olfactory discrimination and neuronal activation of the olfactory system in mice.Mol Autism. 2019 Feb 11;10:5. doi: 10.1186/s13229-019-0257-5. eCollection 2019. Mol Autism. 2019. PMID: 30792833 Free PMC article.
-
A Subtype of Olfactory Bulb Interneurons Is Required for Odor Detection and Discrimination Behaviors.J Neurosci. 2016 Aug 3;36(31):8210-27. doi: 10.1523/JNEUROSCI.2783-15.2016. J Neurosci. 2016. PMID: 27488640 Free PMC article.
-
Functional mapping of odor-activated neurons in the olfactory bulb.Chem Senses. 1995 Apr;20(2):271-82. doi: 10.1093/chemse/20.2.271. Chem Senses. 1995. PMID: 7583022 Review.
-
Zinc as a Neuromodulator in the Central Nervous System with a Focus on the Olfactory Bulb.Front Cell Neurosci. 2017 Sep 21;11:297. doi: 10.3389/fncel.2017.00297. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29033788 Free PMC article. Review.
Cited by
-
Chemogenetic Tools for Causal Cellular and Neuronal Biology.Physiol Rev. 2018 Jan 1;98(1):391-418. doi: 10.1152/physrev.00009.2017. Physiol Rev. 2018. PMID: 29351511 Free PMC article. Review.
-
Mechanistic Resolution Required to Mediate Operant Learned Behaviors: Insights from Neuronal Ensemble-Specific Inactivation.Front Neural Circuits. 2017 Apr 24;11:28. doi: 10.3389/fncir.2017.00028. eCollection 2017. Front Neural Circuits. 2017. PMID: 28484375 Free PMC article.
-
Medial Parabrachial Nucleus Is Essential in Controlling Wakefulness in Rats.Front Neurosci. 2021 Mar 25;15:645877. doi: 10.3389/fnins.2021.645877. eCollection 2021. Front Neurosci. 2021. PMID: 33841086 Free PMC article.
-
Chemogenetics: Beyond Lesions and Electrodes.Neurosurgery. 2021 Jul 15;89(2):185-195. doi: 10.1093/neuros/nyab147. Neurosurgery. 2021. PMID: 33913505 Free PMC article. Review.
-
Molecular Tools for Targeted Control of Nerve Cell Electrical Activity. Part II.Acta Naturae. 2021 Oct-Dec;13(4):17-32. doi: 10.32607/actanaturae.11415. Acta Naturae. 2021. PMID: 35127143 Free PMC article.
References
-
- Barber D. L., Blackburn T. P., Greenwood D. T. (1973). An automatic apparatus for recording rotational behavior in rats with brain lesions. Physiol. Behav. 11, 117–120. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

