Cortical Plasticity and Olfactory Function in Early Blindness
- PMID: 27625596
- PMCID: PMC5003898
- DOI: 10.3389/fnsys.2016.00075
Cortical Plasticity and Olfactory Function in Early Blindness
Abstract
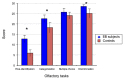
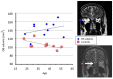
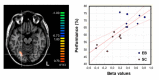
Over the last decade, functional brain imaging has provided insight to the maturation processes and has helped elucidate the pathophysiological mechanisms involved in brain plasticity in the absence of vision. In case of congenital blindness, drastic changes occur within the deafferented "visual" cortex that starts receiving and processing non visual inputs, including olfactory stimuli. This functional reorganization of the occipital cortex gives rise to compensatory perceptual and cognitive mechanisms that help blind persons achieve perceptual tasks, leading to superior olfactory abilities in these subjects. This view receives support from psychophysical testing, volumetric measurements and functional brain imaging studies in humans, which are presented here.
Keywords: congenital blindness; cross-modal plasticity; functional neuroimaging; olfaction; olfactory perception; visual deprivation.
Figures

Comment in
-
Commentary: Cortical Plasticity and Olfactory Function in Early Blindness.Front Hum Neurosci. 2017 Jan 9;10:689. doi: 10.3389/fnhum.2016.00689. eCollection 2016. Front Hum Neurosci. 2017. PMID: 28119592 Free PMC article. No abstract available.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

