Rapid and Sensitive Detection of Didymella bryoniae by Visual Loop-Mediated Isothermal Amplification Assay
- PMID: 27625648
- PMCID: PMC5003822
- DOI: 10.3389/fmicb.2016.01372
Rapid and Sensitive Detection of Didymella bryoniae by Visual Loop-Mediated Isothermal Amplification Assay
Abstract
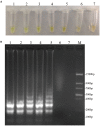
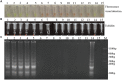
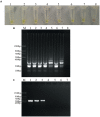
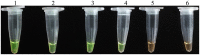
Didymella bryoniae is a pathogenic fungus that causes gummy stem blight (GSB) in Cucurbitaceae crops (e.g., cantaloupe, muskmelon, cucumber, and watermelon). GSB produces lesions on the stems and leaves, and can also be spread by seeds. Here, we developed a rapid, visual, and sensitive loop-mediated amplification (LAMP) assay for D. bryoniae detection based on sequence-characterized amplified regions (GenBank accession nos GQ872461 and GQ872462) common to the two random amplification of polymorphic DNA group genotypes (RGI and RGII) of D. bryoniae; ideal conditions for detection were optimized for completion in 45 min at 63°C. The sensitivity and specificity of the LAMP assay were further analyzed in comparison with those of a conventional polymerase chain reaction (PCR). The sensitivity of the LAMP assay was 1000-fold higher than that of conventional PCR with a detection limit of 0.1 fg μL(-1) of targeted DNA. The LAMP assay could be accomplished in about 45 min, with the results visible to the naked eye. The assay showed high specificity in discriminating all D. bryoniae isolates from seven other fungal pathogens that occur in Cucurbitaceae crops. The LAMP assay also detected D. bryoniae infection in young muskmelon leaves with suspected early symptoms of GSB disease. Hence, the technique has great potential for developing rapid and sensitive visual detection methods for the D. bryoniae pathogen in crops and seeds. This method has potential application in early prediction of disease and reducing the risk of epidemics.
Keywords: Didymella bryoniae; gummy stem blight; loop-mediated isothermal amplification; muskmelon; primer design.
Figures



References
-
- Duan Y., Ge C., Zhang X., Wang J., Zhou M. (2014a). A rapid detection method for the plant pathogen Sclerotinia sclerotiorum based on loop-mediated isothermal amplification (LAMP). Australas. Plant Pathol. 43 61–66. 10.1007/s13313-013-0239-6 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

