The Missing Electrostatic Interactions Between DNA Substrate and Sulfolobus solfataricus DNA Photolyase: What is the Role of Charged Amino Acids in Thermophilic DNA Binding Proteins?
- PMID: 27626127
- PMCID: PMC7301758
- DOI: 10.1021/acs.jpcb.6b07201
The Missing Electrostatic Interactions Between DNA Substrate and Sulfolobus solfataricus DNA Photolyase: What is the Role of Charged Amino Acids in Thermophilic DNA Binding Proteins?
Abstract
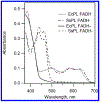
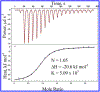
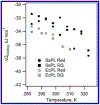
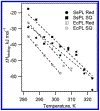
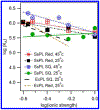
DNA photolyase can be used to study how a protein with its required cofactor has adapted over a large temperature range. The enzymatic activity and thermodynamics of substrate binding for protein from Sulfolobus solfataricus were directly compared to protein from Escherichia coli. Turnover numbers and catalytic activity were virtually identical, but organic cosolvents may be necessary to maintain activity of the thermophilic protein at higher temperatures. UV-damaged DNA binding to the thermophilic protein is less favorable by ∼2 kJ/mol. The enthalpy of binding is ∼10 kJ/mol less exothermic for the thermophile, but the amount and type of surface area buried upon DNA binding appears to be somewhat similar. The most important finding was observed when ionic strength studies were used to separate binding interactions into electrostatic and nonelectrostatic contributions; DNA binding to the thermophilic protein appears to lack the electrostatic contributions observed with the mesophilic protein.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Sancar A Structure and Function of DNA Photolyase and Cryptochrome Blue-Light Photoreceptors. Chem. Rev 2003, 103, 2203–2237. - PubMed
-
- Müller M; Carell T Structural Biology of DNA Photolyases and Cryptochromes. Curr. Opin. Struct. Biol 2009, 19, 277–285. - PubMed
-
- Brock TD; Brock KM; Belly RT; Weiss RL Sulfolobus: A New Genus of Sulfur-Oxidizing Bacteria Living at Low pH and High Temperature. Arch. Microbiol 1972, 84, 54–68. - PubMed
-
- Fujihashi M; Numoto N; Kobayashi Y; Mizushima A; Tsujimura M; Nakamura A; Kawarabayasi Y; Miki K Crystal Structure of Archael Photolyase From Sulfolobus Tokodaii with Two FAD Molecules: Implication of a Novel Light-Harvesting Cofactor. J. Mol. Biol 2007, 365, 903–910. - PubMed
-
- EMBL-EBI EMBOSS Matcher. http://www.ebi.ac.uk/Tools/psa/emboss_matcher/ (accessed August 29, 2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

